29883-15-6
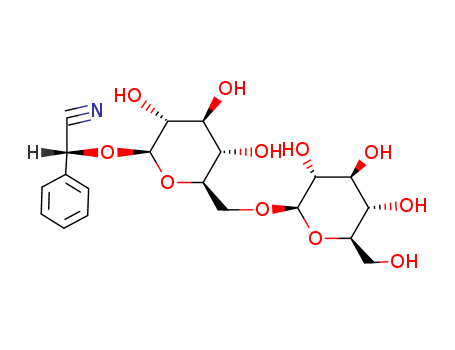
- Product Name:Amygdalin
- Molecular Formula:C20H27NO11
- Purity:99%
- Molecular Weight:457.434
Product Details;
CasNo: 29883-15-6
Molecular Formula: C20H27NO11
Appearance: white fine crystalline powder
Chemical plants supply high-quality Amygdalin 29883-15-6 in bulk
- Molecular Formula:C20H27NO11
- Molecular Weight:457.434
- Appearance/Colour:white fine crystalline powder
- Vapor Pressure:3.35E-23mmHg at 25°C
- Melting Point:223-226 °C(lit.)
- Refractive Index:-40 ° (C=2, H2O)
- Boiling Point:743.3 °C at 760 mmHg
- PKA:12.69±0.70(Predicted)
- Flash Point:403.3 °C
- PSA:202.32000
- Density:1.59 g/cm3
- LogP:-3.10802
Amygdalin(Cas 29883-15-6) Usage
|
Uses and Synthetic Methods of Amygdalin |
Amygdalin is a β-cyano-glycoside in the bitter almond that is bound to cyano (CN), which releases free cyano groups after eating the bitter almond, so that the food is poisoned. Amygdalin is a product of the metabolism of phenylalanine in the bitter almond. Amygdalin has β-glucosidase and amygdalinase (oxynitrilase): the former catalyzed amygdalin into two molecules of glucose and one molecule of amygdalenone through hydrolysis; the later catalyzes mandelonitrile into almond nitrile cyanide (HCN) and benzaldehyde through hydrolysis. Amygdalin exists in seeds, such as almonds. Many plant root cells contain glycosides, with no toxic effect when in sugar type. Glycosides hydrolysis produce toxic substances leading to cell death. Mandelic glycosides contained in peach root is a glycoside. Amylose hydrolysis produce two kinds of plant toxins-hydrogen cyanide and benzaldehyde. Peeling root lesion formation and necrosis occurred in the vicinity of the stabbed nematode but not in contact zone; in addition, in vitro tests have proved that the pratylenchus penetrans can make amygdalin hydrolysis. As a cyanide containing glucoside, it can be used as a substrate for such as maltase, almond casein and β-glucosidase identification, differentiation and characterization. |
|
Toxicity classification |
Highly toxic |
|
Acute toxicity |
Acute toxicity Oral-Rat LD50: 522 mg/kg; Oral-mouse LD50: 443 mg/kg |
|
Flammability Hazardous properties |
Combustible; producing toxic nitrogen oxides when heated. |
|
Storage and transportation |
Ventilation, low temperature, drying |
|
Fire extinguishing agent |
Dry powder, foam, sand, carbon dioxide |
|
World Health Organization (WHO) |
Laetrile, which consists mainly of amydgalin, a glycoside extracted from the kernels of apricots, peaches and other fruits, has been available for over 30 years in preparations purporting to be beneficial in the treatment of cancer. Although there is no evidence that these are efficacious, preparations continued to be widely used and, until the late 1970s, they were considered to be harmless. However, oral dosage forms, which may be broken down in the gut to hydrogen cyanide, have subsequently been shown to be potentially lethal. This has resulted in restrictive regulatory measures in several countries. |
|
Biochem/physiol Actions |
Cyanogenic glycoside that is a component of bitter almonds and apricot pits. There is no scientific evidence that amygdalin itself is an effective anti-cancer agent. Recent studies using β?glucoside linked to a tumor-associated monoclonal antibody to release cyanide at the tumor cell has shown significant cytotoxicity. |
|
Safety Profile |
Human poison by ingestion(infant data). Poison experimentally by ingestion. Anexperimental teratogen. Mutation data reported. Whenheated to decomposition it emits toxic fumes of NOx. |
|
Purification Methods |
D-Amygdalin recrystallises from water as the trihydrate, or from EtOH. It is present in bitter almonds. [Smith Chem Ber 64 1115 1931, Beilstein 17/8 V 188.] |
|
Category |
Toxic substances |
|
Definition |
ChEBI: An amygdalin in which the stereocentre on the cyanohydrin function has R-configuration. |
|
General Description |
This substance is a primary reference substance with assigned absolute purity (considering chromatographic purity, water, residual solvents, inorganic impurities). The exact value can be found on the certificate. Produced by PhytoLab GmbH & Co. KG |
InChI:InChI=1/C20H27NO11/c21-6-10(9-4-2-1-3-5-9)30-20-18(28)16(26)14(24)12(32-20)8-29-19-17(27)15(25)13(23)11(7-22)31-19/h1-5,10-20,22-28H,7-8H2/t10-,11+,12+,13+,14+,15-,16-,17+,18+,19+,20+/m0/s1
29883-15-6 Relevant articles
Synthesis and Biological Evaluation of Cyanogenic Glycosides
Yashunsky, Dmitry V.,Kulakovskaya, Ekaterina V.,Kulakovskaya, Tatiana V.,Zhukova, Olga S.,Kiselevskiy, Mikhail V.,Nifantiev, Nikolay E.
, p. 460 - 474 (2015/12/23)
An efficient procedure for the synthesis...
Aromatic glucosides from the seeds of Prunus davidiana
Chen, Xiao-Yan,Wang, Hong-Qing,Zhang, Ting,Liu, Chao,Kang, Jie,Chen, Ruo-Yun,Yu, De-Quan
, p. 1528 - 1534 (2013/10/22)
Chemical investigation of the seeds of P...
Study on the Prevention of Racemization of Amygdalin
Takayama, Yuzi,Kawai, Satoshi
, p. 778 - 781 (2007/10/02)
Studies were conducted to find suitable ...
29883-15-6 Process route
-
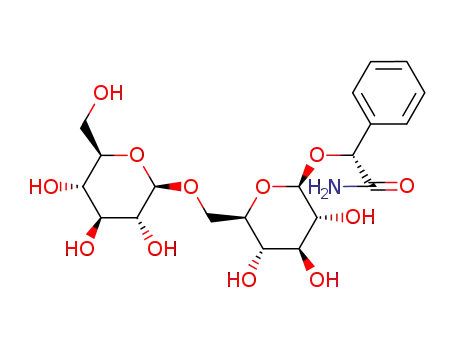
-
66427-91-6
(R)-(6-O-β-D-glucopyranosyl-β-D-glucopyranosyloxy)(phenyl)acetamide

-
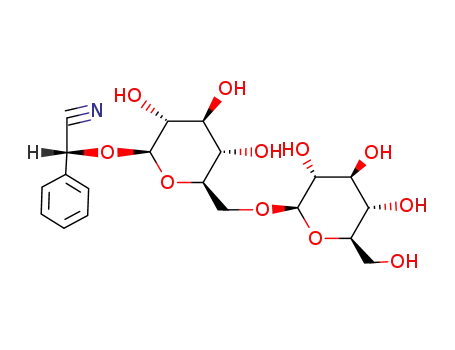
-
29883-15-6,672-72-0
amygdalin
| Conditions | Yield |
|---|---|
|
With
pyridine; trifluoroacetic anhydride;
In
1,4-dioxane;
at 20 ℃;
for 2h;
|
20 mg |
-
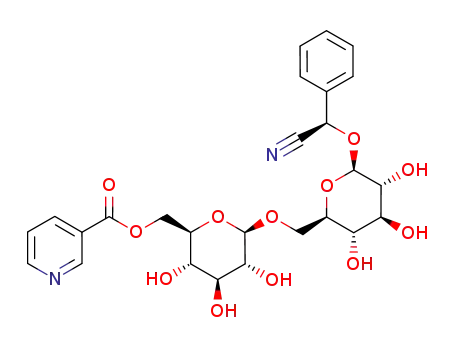
-
prupersin E

-

-
29883-15-6,672-72-0
amygdalin
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
methanol; water;
at 20 ℃;
for 4h;
|
1 mg |
29883-15-6 Upstream products
-
66427-91-6

(R)-(6-O-β-D-glucopyranosyl-β-D-glucopyranosyloxy)(phenyl)acetamide
-
29883-15-6
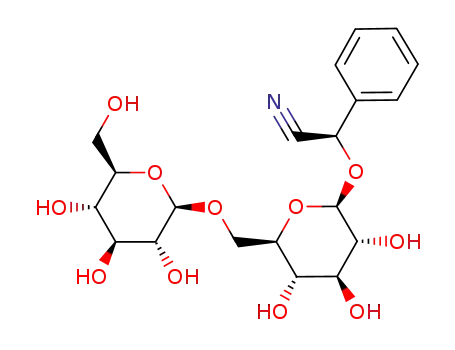
amygdalin
29883-15-6 Downstream products
-
87-74-1
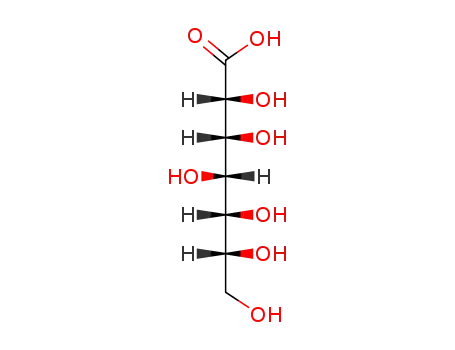
D-glucoheptonic acid
-
74-90-8

hydrogen cyanide
-
532-28-5
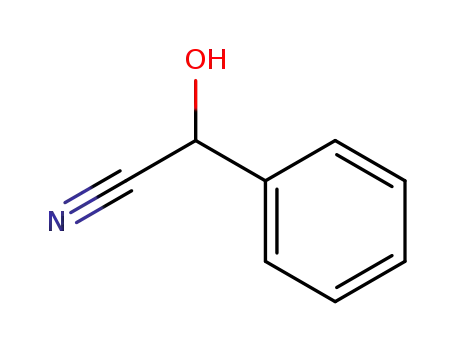
(RS)-mandelonitrile
-
10020-96-9
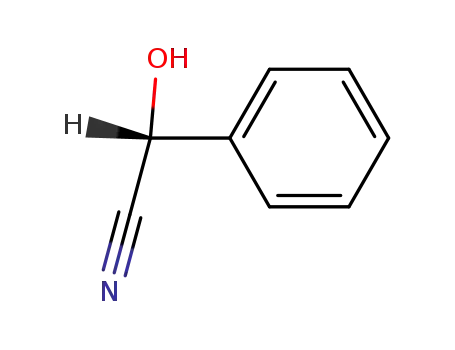
(R)-Mandelonitrile
Relevant Products
-
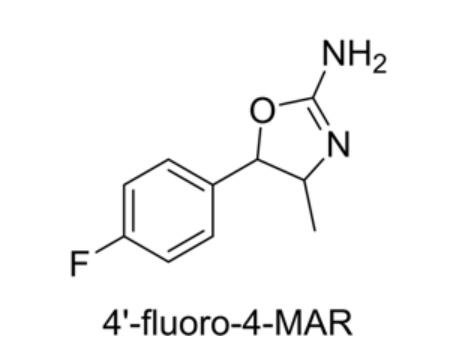
para-fluoro Methylaminorex
CAS:1364933-64-1
-
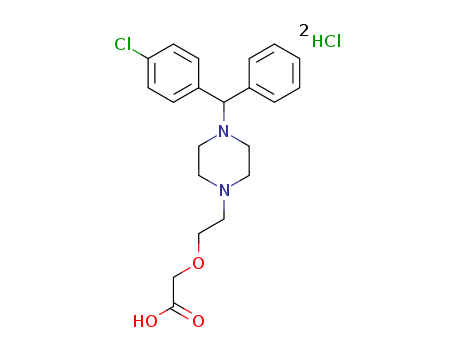
Cetirizine dihydrochloride
CAS:83881-52-1
-
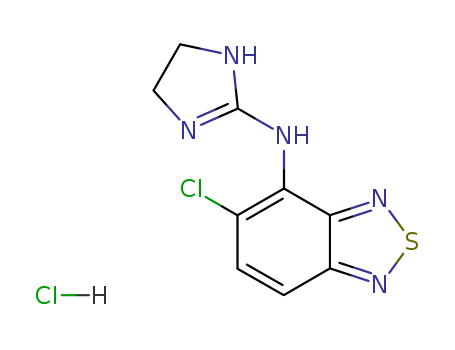
Tizanidine hydrochloride
CAS:64461-82-1