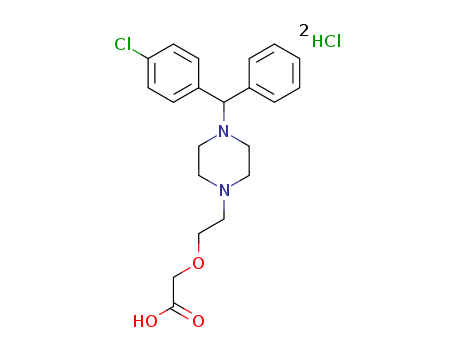
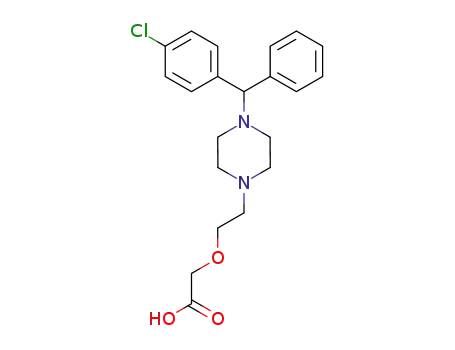
83881-52-1
- Product Name:Cetirizine dihydrochloride
- Molecular Formula:C21H25ClN2O3.2(HCl)
- Purity:99%
- Molecular Weight:461.816
Product Details;
CasNo: 83881-52-1
Molecular Formula: C21H25ClN2O3.2(HCl)
Appearance: Crystalline solid
Quality Manufacturer Supply High Purity 99% Cetirizine dihydrochloride 83881-52-1 with Reasonable Price
- Molecular Formula:C21H25ClN2O3.2(HCl)
- Molecular Weight:461.816
- Appearance/Colour:Crystalline solid
- Vapor Pressure:1.39E-12mmHg at 25°C
- Melting Point:110-115 °C
- Boiling Point:542.1 °C at 760 mmHg
- Flash Point:281.6 °C
- PSA:53.01000
- Density:1.237 g/cm3
- LogP:3.82600
Cetirizine hydrochloride(Cas 83881-52-1) Usage
|
Application |
Cetirizine Hydrochloride may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by various chromatography techniques. |
|
Indications |
Cetirizine HCl (Zyrtec) is the carboxylic acid metabolite of hydroxyzine. It is a selective, peripheral H1 receptor antagonist. It is a long-lasting antihistamine. It does not appear to have the same adverse cardiac effects as the other nonsedating H1 antihistamines; however, additional data are required. Indicated for allergic rhinitis and chronic urticaria. |
|
Definition |
ChEBI: Cetirizine hydrochloride is a diarylmethane. |
|
Manufacturing Process |
Preparation of 2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]- ethoxy]acetic acid (cetirizine). To a mixture of 50 g 2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]- ethanol and 225 ml of tert-butanol at 45°C under a nitrogen was added 21 g tert-BuOK. The temperature was raised to 75-80°C and the mixture was kept at this temperature. After 45 min was added 11 g sodium chloracetate; after 1.5 hour was added 5.2 g tert-BuOK; after 2 hours was added 5.64 g sodium chloracetate; after 2.5 hours was added 1.9 g tert-BuOK; after 3 hours was added 1.9 g sodium chloracetate; after 3.5 hours was added 0.8 g tert-BuOK; and after 4 hours was added 1.13 g sodium chloracetate. Then about 150 ml tert-butanol was distilled of, 190 ml of water was added and the distillation of tert-butanol was continued until the temperature of the vapour reaches 100°C. To the reaction mixture was added 60 ml of water and 8 ml concentrated hydrochloric acid to pH 8. Unreacted 2-[4-[(4-chlorophenyl) phenylmethyl]-1-piperazinyl]-ethanol was extracted with diethyl ether. The aqueous phase was acidified to pH 5 by addition of hydrochloric acid and extracted with dichloromethane (200 ml x 3). The extract was dried over MgSO4, filtered and concentrated in a rotary evaporator. An obtained oil was allowed to crystallize by addition of 150 ml of 2-butanone, yields of 2-[4-[(4- chlorophenyl)phenylmethyl]-1-piperazinyl]-ethoxy]acetic acid 55.5%, M.P. 146-148°C. 32.7 g 2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]-ethoxy]acetic acid was suspended in a mixture of 125 ml of water and 13.8 ml 37% aqueous hydrochloric acid. The mixture was concentrated in a rotary evaporator. An obtained oil was allowed to crystallize by addition of 245 ml of 2-butanone, yields of 2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]-ethoxy]acetic acid dihydrochloride 88%, M.P. 228.22°C. |
|
Therapeutic Function |
Antihistaminic; Antiallergic |
|
General Description |
Cetirizine hydrochloride, a second generation antihistaminic drug, is one of the carboxylated metabolites of hydroxyzine that can typically bind to histamine H1 receptor. It is effective against diseases such as urticaria, angioedema, allergies and hay fever. |
|
Biological Activity |
Histamine H 1 receptor antagonist that displays selectivity over other receptors at concentrations up to 10 μ M. A non-sedating antihistamine that inhibits histamine release and eosinophil chemotaxis during secondary phase allergic response. Inhibits activation of eosinophils, neutrophils and monocytes in vivo . |
|
Biochem/physiol Actions |
Cetirizine hydrochloride is an orally active and selective H1-receptor antagonist. Antihistaminic; Piperazines. Non-sedating type histamine H1-receptor antagonist; major metabolite of hydroxyzine. Pharmacological activity resides primarily in the (R)-isomer. |
|
Veterinary Drugs and Treatments |
Cetirizine is a H1 receptor blocking antihistamine agent that may be useful for the adjunctive treatment of histamine-mediated pruritic conditions in dogs or cats. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Antivirals: concentration possibly increased by ritonavir. |
|
Metabolism |
Cetirizine does not undergo extensive first pass metabolism. About two thirds of the dose is excreted unchanged in urine. |
|
Dosage forms |
5 to 10mg daily. |
InChI:InChI=1/C21H25ClN2O3.2ClH/c22-19-8-6-18(7-9-19)21(17-4-2-1-3-5-17)24-12-10-23(11-13-24)14-15-27-16-20(25)26;;/h1-9,21H,10-16H2,(H,25,26);2*1H
83881-52-1 Relevant articles
A synthesis process of cetirizine hydrochloride
-
Paragraph 0074; 0075; 0076; 0077, (2017/03/08)
A new technology for synthesizing cetiri...
New manufacturing procedure of cetirizine
Reiter, Jozsef,Trinka, Peter,Bartha, Ferenc L.,Pongo, Laszlo,Volk, Balazs,Simig, Gyula
, p. 1279 - 1282 (2012/09/08)
A new procedure for the manufacture of c...
Process for obtaining cetirizine dihydrochloride
-
Page/Page column 3-4, (2009/02/11)
Process for the synthesis of cetirizine ...
Crystalline cetirizine monohydrochloride
-
Page 5-6, (2008/06/13)
A novel crystalline form of cetirizine m...
83881-52-1 Process route
-
-
83881-51-0
cetirizine

-
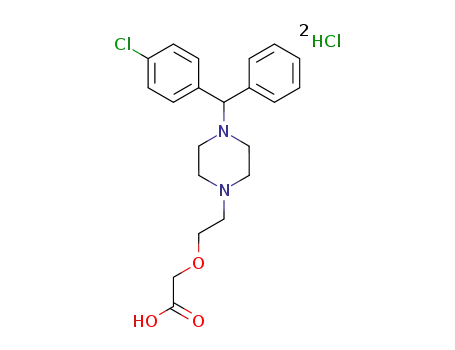
-
83881-52-1,130018-87-0,163837-48-7
cetirizine dihydrochloride
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water; acetone;
at 20 - 50 ℃;
for 2h;
|
87.9% |
|
With
hydrogenchloride;
In
water;
pH=8;
|
|
|
With
hydrogenchloride;
In
ethyl acetate; isopropyl alcohol;
at 25 - 35 ℃;
for 1 - 2h;
pH=2;
|
-
-
770697-99-9
C21H25ClN2O2

-

-
83881-52-1,130018-87-0,163837-48-7
cetirizine dihydrochloride
| Conditions | Yield |
|---|---|
|
C21H25ClN2O2;
With
potassium dihydrogenphosphate; phosphoric acid; dihydrogen peroxide;
In
dichloromethane; dimethyl sulfoxide;
at 5 - 25 ℃;
for 8h;
pH=4 - 5;
With
hydrogenchloride;
In
butanone;
at 60 ℃;
|
86% |
83881-52-1 Upstream products
-
83881-37-2
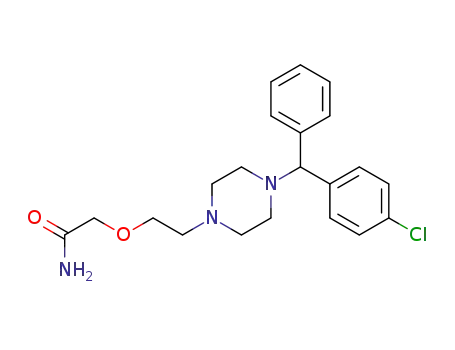
2-(2-(4-((4-chlorophenyl)(phenyl)methyl)piperazin-1-yl)ethoxy)acetamide
-
83881-51-0

cetirizine
-
83881-51-0
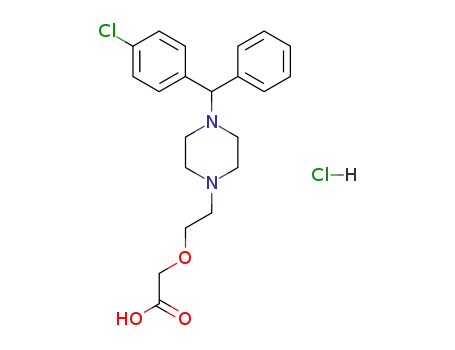
cetirizine hydrochloride
-
343781-29-3
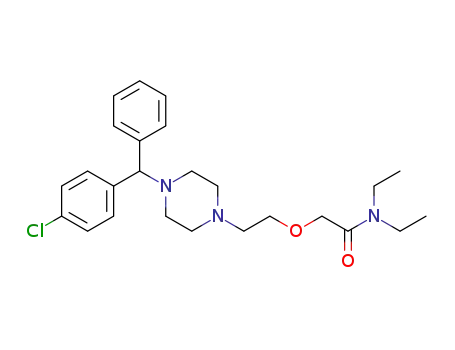
(RS)-N,N-diethyl-{2-[4-(α-phenyl-p-chloro-benzyl)piperazin-1-yl]ethoxy}-acetamide
83881-52-1 Downstream products
-
83881-51-0

cetirizine
-
83881-51-0

cetirizine hydrochloride
-
83881-46-3
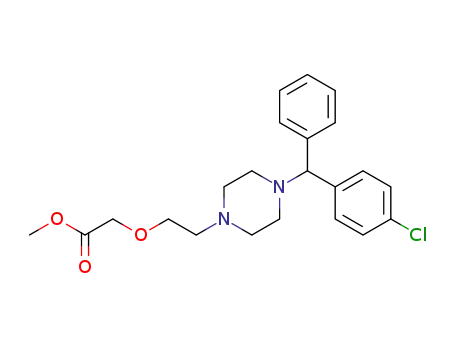
methyl 2-[2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy]-acetate
-
1080673-10-4
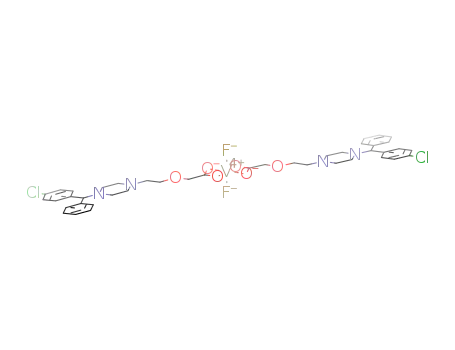
difluorovanadium(IV)(2-[4-[(4-chlorophenyl)phenylmethyl]-1-piperazinyl]ethoxy acetate)
Relevant Products
-
Aripiprazole
CAS:129722-12-9
-
Amygdalin
CAS:29883-15-6