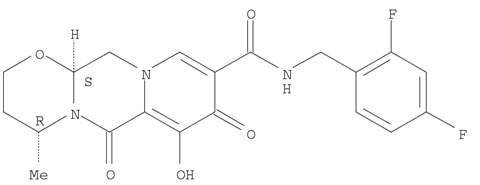
1051375-16-6
- Product Name:Dolutegravir; DTG; GSK1349572
- Molecular Formula:C20H19F2N3O5
- Purity:99%
- Molecular Weight:419.13
Product Details;
CasNo: 1051375-16-6
Molecular Formula: C20H19F2N3O5
Appearance: White to Pale Yellow Solid
Chinese Manufacturer supply Dolutegravir; DTG; GSK1349572 1051375-16-6 in stock with high standard
- Molecular Formula:C20H19F2N3O5
- Molecular Weight:419.13
- Appearance/Colour:White to Pale Yellow Solid
- Melting Point:188-192°C
- Boiling Point:668.958 °C at 760 mmHg
- PKA:4.50±1.00(Predicted)
- Flash Point:358.373 °C
- PSA:100.87000
- Density:1.532 g/cm3
- LogP:1.68160
1051375-16-6 Relevant articles
7-Step Flow Synthesis of the HIV Integrase Inhibitor Dolutegravir
Ziegler, Robert E.,Desai, Bimbisar K.,Jee, Jo-Ann,Gupton, B. Frank,Roper, Thomas D.,Jamison, Timothy F.
, p. 7181 - 7185 (2018)
Dolutegravir (DTG), an important active ...
Practical and Scalable Synthetic Method for Preparation of Dolutegravir Sodium: Improvement of a Synthetic Route for Large-Scale Synthesis
Aoyama, Yasunori,Hakogi, Toshikazu,Fukui, Yuki,Yamada, Daisuke,Ooyama, Takao,Nishino, Yutaka,Shinomoto, Shoji,Nagai, Masahiko,Miyake, Naoki,Taoda, Yoshiyuki,Yoshida, Hiroshi,Yasukata, Tatsuro
, p. 558 - 564 (2019)
A practical and scalable synthetic metho...
Identification and Control of Critical Process Impurities: An Improved Process for the Preparation of Dolutegravir Sodium
Sankareswaran, Srimurugan,Mannam, Madhavarao,Chakka, Veerababu,Mandapati, Srirami Reddy,Kumar, Pramod
, p. 1461 - 1468 (2016)
A four-stage manufacturing route for the...
Six-Step Gram-Scale Synthesis of the Human Immunodeficiency Virus Integrase Inhibitor Dolutegravir Sodium
Dietz, Jule-Philipp,Lucas, Tobias,Gro?, Jonathan,Seitel, Sebastian,Brauer, Jan,Ferenc, Dorota,Gupton, B. Frank,Opatz, Till
, p. 1898 - 1910 (2021)
A short and practical synthesis for prep...
Synthesis of two diastereomeric impurities of a fluorinated antiretroviral drug dolutegravir
Amasa, Srinivasulu Reddy,Garrepalli, Sailaja,Gudipati, Ramesh,Pal, Manojit,Ravindhranath, Kunta
, (2022/01/10)
The study of drug impurities constitutes...
NOVEL PYRROLE AND PYRIDONE DERIVATIVES AND USES THEREOF
-
Page/Page column 41, (2020/05/21)
Pyrrole and pyridine derivatives and met...
CONTINUES FLOW PROCESS FOR THE PREPARATION OF ACTIVE PHARMACEUTICAL INGREDIENTS - POLYCYCLIC CARBAMOYL PYRIDONE DERIVATIVES AND INTERMEDIATES THEREOF
-
Page/Page column 34; 35; 36; 38; 39, (2019/09/04)
The present invention discloses continue...
1051375-16-6 Process route
-
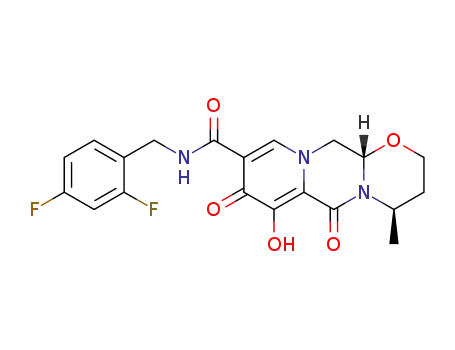
![(4R,12aS)-N-(2,4-difluorobenzyl)-7-methyloxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido [1',2',:4,5]pyrazino[2,1-b] [1,3]oxazine-9-carboxamide](/upload/2025/4\5c3a2554-12f6-47df-90b1-3d341eb69a92.png)
-
1335210-35-9
(4R,12aS)-N-(2,4-difluorobenzyl)-7-methyloxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido [1',2',:4,5]pyrazino[2,1-b] [1,3]oxazine-9-carboxamide

-
-
1051375-16-6,1309560-49-3
dolutegravir
| Conditions | Yield |
|---|---|
|
With
lithium bromide;
In
tetrahydrofuran; methanol; isopropyl alcohol;
at 60 ℃;
for 6h;
Solvent;
Large scale;
|
92% |
|
With
water; lithium bromide;
In
tetrahydrofuran;
at 100 ℃;
under 5171.62 Torr;
Temperature;
Flow reactor;
|
89% |
|
With
magnesium bromide;
In
acetonitrile;
at 50 ℃;
for 30h;
|
43% |
|
With
lithium bromide;
In
isopropyl alcohol;
at 70 - 80 ℃;
for 15h;
|
1.5 g |
|
(4R,12aS)-N-(2,4-difluorobenzyl)-7-methyloxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido [1',2',:4,5]pyrazino[2,1-b] [1,3]oxazine-9-carboxamide;
With
magnesium dibromide;
In
acetonitrile;
at 50 - 52 ℃;
for 8h;
With
hydrogenchloride;
In
dichloromethane; water;
|
67 g |
|
With
magnesium bromide hexahydrate;
at 80 ℃;
for 2h;
|
-
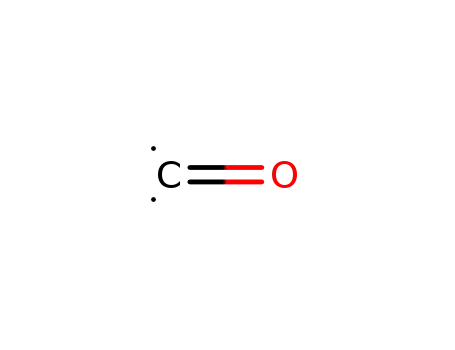
-
201230-82-2
carbon monoxide

-
![(4R,12aS)-7-(benzyloxy)-9-bromo-4-methyl-3,4,12,12atetrahydro-2H-pyrido [1′,2′:4,5]pyrazino[2,1-b][1,3]oxazine-6,8-dione](/upload/2025/4\1d2b845f-236e-4004-a946-6857d22ed835.png)
-
1206102-10-4
(4R,12aS)-7-(benzyloxy)-9-bromo-4-methyl-3,4,12,12atetrahydro-2H-pyrido [1′,2′:4,5]pyrazino[2,1-b][1,3]oxazine-6,8-dione

-
-
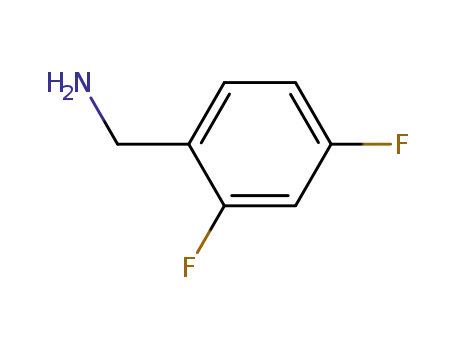
72235-52-0
2,4-Difluoro-benzylamine

-

-
1051375-16-6,1309560-49-3
dolutegravir
| Conditions | Yield |
|---|---|
|
carbon monoxide; (4R,12aS)-7-(benzyloxy)-9-bromo-4-methyl-3,4,12,12atetrahydro-2H-pyrido [1′,2′:4,5]pyrazino[2,1-b][1,3]oxazine-6,8-dione; 2,4-Difluoro-benzylamine;
With
tetrakis(triphenylphosphine) palladium(0); triethylamine;
In
dimethyl sulfoxide;
at 180 ℃;
for 5h;
under 4500.45 Torr;
With
hydrogenchloride;
In
water;
|
75% |
1051375-16-6 Upstream products
-
1206102-11-5
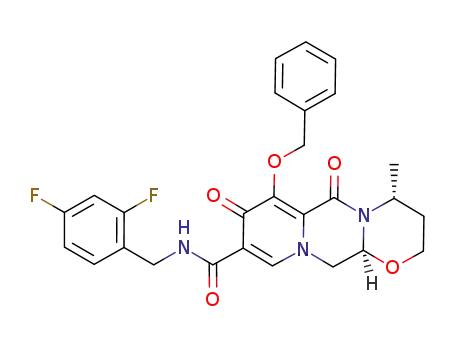
(4R,12aS)-7-(benzyloxy)-N-(2,4-difluorobenzyl)-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1′,2’:4,5]-pyrazino[2,1-b][1,3]oxazine-9-carboxamide
-
1246616-70-5
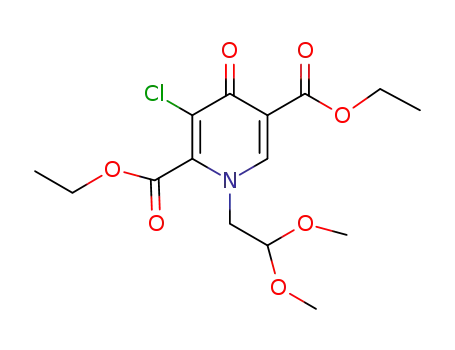
C15H20ClNO7
-
1246616-71-6
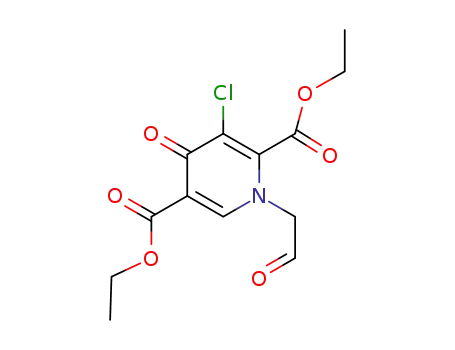
C13H14ClNO6
-
1246616-72-7
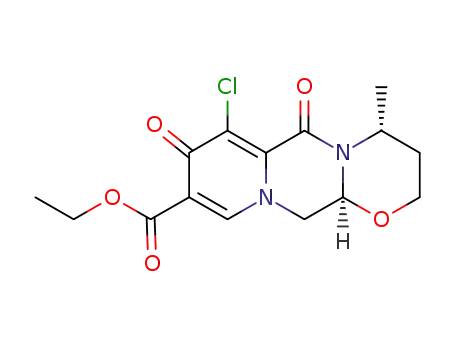
C15H17ClN2O5
1051375-16-6 Downstream products
-
1051375-19-9
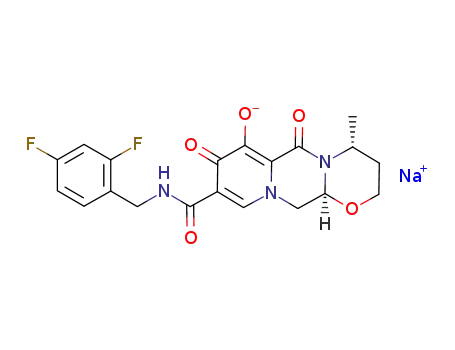
(4R,12aS)-N-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido[1′,2′:4,5]pyrazino-[2,1-b][1,3]oxazine-9-carboxamide sodium salt
Relevant Products
-
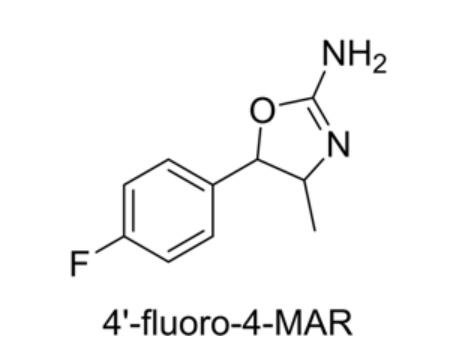
para-fluoro Methylaminorex
CAS:1364933-64-1
-
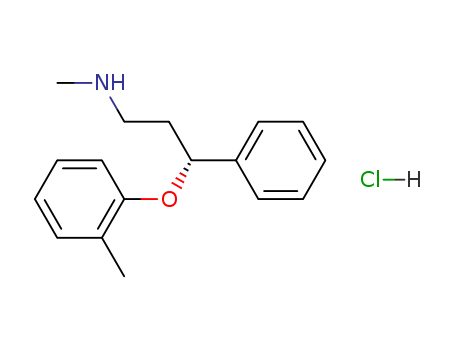
Atomoxetine hydrochloride hcl
CAS:82248-59-7
-
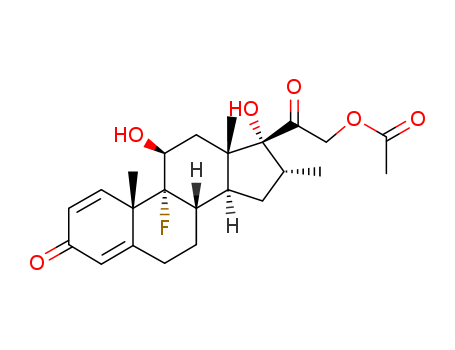
Dexamethasone Acetate
CAS:1177-87-3