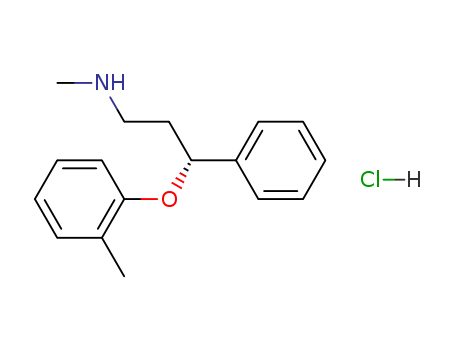
82248-59-7
- Product Name:Atomoxetine hydrochloride hcl
- Molecular Formula:C17H22ClNO
- Purity:99%
- Molecular Weight:291.821
Product Details;
CasNo: 82248-59-7
Molecular Formula: C17H22ClNO
Appearance: solid
Reputable factory supply Atomoxetine hydrochloride hcl 82248-59-7 in bulk at low price
- Molecular Formula:C17H22ClNO
- Molecular Weight:291.821
- Appearance/Colour:solid
- Vapor Pressure:2.95E-06mmHg at 25°C
- Melting Point:167-169oC
- Boiling Point:389 °C at 760 mmHg
- PKA:10.13(at 25℃)
- Flash Point:164.1 °C
- PSA:21.26000
- LogP:4.91750
Atomoxetine hydrochloride(Cas 82248-59-7) Usage
|
Biological Activity |
Potent and selective noradrenalin re-uptake inhibitor (K i values are 5, 77 and 1451 nM for inhibition of radioligand binding to human NET, SERT and DAT respectively). Displays minimal affinity for a range of other neurotransmitter receptors and transporters (K i > 1 μ M). Antidepressant. |
|
Synthesis |
The 3-aryloxy substituent was introduced utilizing a chiral alcohol by either the Mitsunobu reaction or by nucleophilic aromatic displacement. Because of the expense and difficulty of the Mitsunobu reaction on large scale, the commercial process adopts the nucleophilic aromatic substitution method. 3- Chloropropiophenone (37) was asymmetrically reduced with borane and catalytic amount of (S)-oxazaborolidine (8) in THF at 0°C to give chiral alcohol 38 in 99% yield and 94% e.e. The chiral alcohol was further purified by recrystallization to greater than 99% e.e.. Subsequent treatment of chloride 38 with excess dimethylamine (40% in water) in ethanol gave dimethylamine alcohol 39 in 90% yield. Alcohol 39 was then subjected to nucleophilic aromatic displacement in the presence of NaH in DMSO with 1- fluoro-2-(t-butylimino)benzene (41), which was prepared in high yield from 2-fluorobenzaldehyde (40). The displacement product 42 was obtained in 98% yield, and the imine 42 was subsequently hydrolyzed with acetic acid in water at low temperature to give the corresponding aldehyde 43 in 96% yield. Sodium borohydride was employed to reduce aldehyde 43 to alcohol in cold methanol and the intermediate alcohol was converted to chloride 44 with thionyl chloride. Chloride 44 was then reduced with zinc metal under acidic conditions to give methyl adduct 45 in 95% yield and 94% e.e. Finally, phenyl chloroformate and triethylamine was used to transform dimethylamine 45 to monomethyl amine, which was subsequently treated with HCl in EtOAc under reflux to give atomoxetin hydrochloride (IV) in 98% yield and 99% e.e. from 45. |
|
Definition |
ChEBI: The hydrochloride salt of atomoxetine. |
|
Brand name |
Strattera (Lilly). |
|
General Description |
Atomoxetine hydrochloride is a nonstimulant used in the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) in children and adults. |
InChI:InChI=1/C17H21NO.ClH/c1-14-8-6-7-11-16(14)19-17(12-13-18-2)15-9-4-3-5-10-15;/h3-11,17-18H,12-13H2,1-2H3;1H
82248-59-7 Relevant articles
Preparation method of cefamoxetine hydrochloride
-
Paragraph 0009; 0018; 0020; 0023; 0026; 0029, (2021/10/27)
The invention discloses a preparation me...
Preparation method of atomoxetine hydrochloride
-
Paragraph 0015, (2020/06/09)
The invention belongs to the technical f...
R-(-)-atomoxetine hydrochloride preparation method
-
Paragraph 0087; 0091-0093; 0097-0099; 0103-0104; 0109, (2019/09/14)
The invention provides an R-(-)-atomoxet...
Preparation method of atomoxetine hydrochloride
-
Paragraph 0041; 0044-0047, (2019/10/15)
The invention relates to the field of me...
82248-59-7 Process route
-
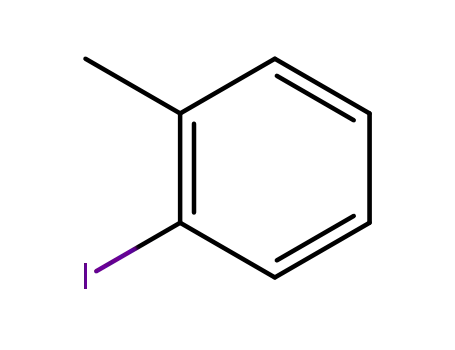
-
615-37-2
ortho-methylphenyl iodide

-
-
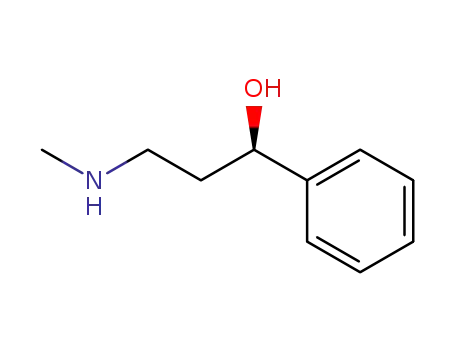
115290-81-8
(R)-N-methyl-3-phenyl-3-hydroxypropylamine

-
-
82248-59-7,82857-40-7
atomoxetine hydrochloride
| Conditions | Yield |
|---|---|
|
ortho-methylphenyl iodide; (R)-N-methyl-3-phenyl-3-hydroxypropylamine;
With
potassium carbonate;
copper(l) iodide;
In
toluene;
at 148 ℃;
for 21h;
Heating / reflux;
With
hydrogenchloride;
In
water;
pH=1 - 2;
With
sodium hydroxide;
In
water;
pH=11 - 12;
|
99% |
|
ortho-methylphenyl iodide; (R)-N-methyl-3-phenyl-3-hydroxypropylamine;
With
potassium carbonate;
copper(l) iodide;
In
toluene;
at 148 ℃;
for 21h;
Heating / reflux;
With
hydrogenchloride;
In
water;
pH=1 - 2;
With
sodium hydroxide;
In
water;
pH=11 - 12;
|
94% |
|
ortho-methylphenyl iodide; (R)-N-methyl-3-phenyl-3-hydroxypropylamine;
With
potassium phosphate;
copper(l) iodide;
In
toluene;
for 24h;
Heating / reflux;
With
hydrogenchloride;
In
water;
pH=1 - 2;
With
sodium hydroxide;
In
water;
pH=12 - 14;
|
82% |
|
ortho-methylphenyl iodide; (R)-N-methyl-3-phenyl-3-hydroxypropylamine;
With
caesium carbonate;
copper(l) iodide;
In
xylenes;
at 130 ℃;
With
hydrogenchloride;
In
tert-butyl methyl ether; isopropyl alcohol; xylenes;
at 0 - 5 ℃;
Product distribution / selectivity;
|
|
|
ortho-methylphenyl iodide; (R)-N-methyl-3-phenyl-3-hydroxypropylamine;
With
potassium carbonate;
copper(l) iodide;
In
xylenes;
for 130h;
With
hydrogenchloride;
In
ethyl acetate; isopropyl alcohol;
at 0 - 5 ℃;
Product distribution / selectivity;
|
|
|
ortho-methylphenyl iodide; (R)-N-methyl-3-phenyl-3-hydroxypropylamine;
With
caesium carbonate;
copper(l) iodide;
In
xylenes;
at 130 ℃;
With
hydrogenchloride;
In
tert-butyl methyl ether; isopropyl alcohol; xylenes;
at 0 - 5 ℃;
Product distribution / selectivity;
|
|
|
ortho-methylphenyl iodide; (R)-N-methyl-3-phenyl-3-hydroxypropylamine;
With
potassium carbonate;
copper(l) iodide;
In
xylenes;
at 130 ℃;
With
hydrogenchloride;
In
water; xylenes;
at 20 ℃;
With
hydrogenchloride; sodium hydroxide;
more than 3 stages;
Product distribution / selectivity;
|
-
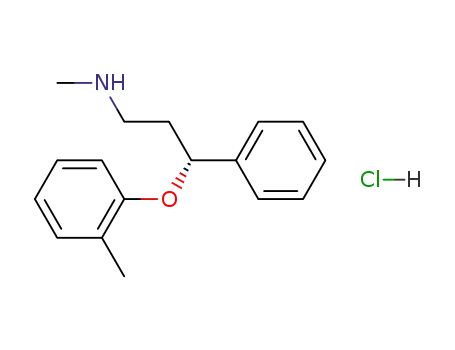
-
R-(-)-atomoxetine-S-(+)-mandelate

-

-
82248-59-7,82857-40-7
atomoxetine hydrochloride
| Conditions | Yield |
|---|---|
|
R-(-)-atomoxetine-S-(+)-mandelate;
With
sodium hydroxide;
In
water;
at 40 - 45 ℃;
for 0.166667h;
Large scale;
With
hydrogenchloride;
In
water;
at 20 ℃;
Large scale;
|
95% |
82248-59-7 Upstream products
-
134619-78-6
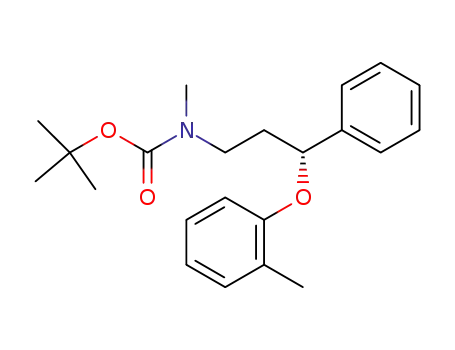
(-)-N-tert-butoxycarbonyl-N-methyl-3-phenyl-3-(2-methylphenoxy)propanamine
-
95-48-7
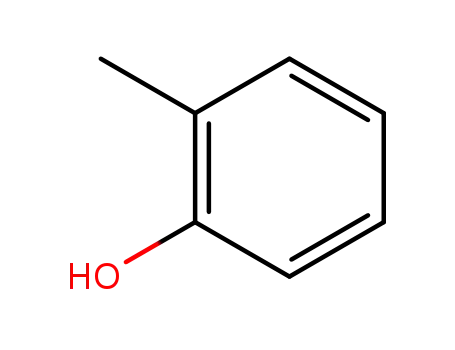
ortho-cresol
-
115290-81-8

(R)-N-methyl-3-phenyl-3-hydroxypropylamine
-
114446-47-8
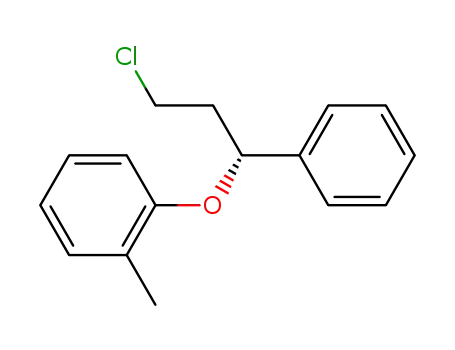
-(-)-1-chloro-3-phenyl-3-(2-methylphenoxy)propane
82248-59-7 Downstream products
-
134619-78-6

(-)-N-tert-butoxycarbonyl-N-methyl-3-phenyl-3-(2-methylphenoxy)propanamine
Relevant Products
-
Phenol,4-[2-(dimethylamino)ethyl]-, hydrochloride (1:1)
CAS:6027-23-2
-
Adrenochrome
CAS:54-06-8
-
Dolutegravir; DTG; GSK1349572
CAS:1051375-16-6