1177-87-3
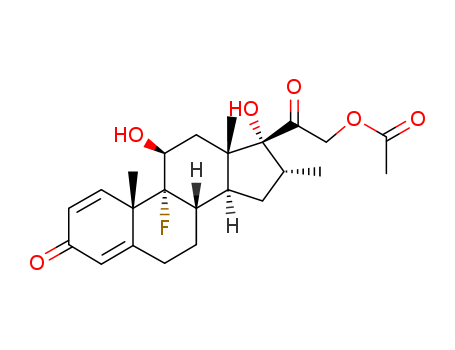
- Product Name:Dexamethasone Acetate
- Molecular Formula:C24H31FO6
- Purity:99%
- Molecular Weight:434.505
Product Details;
CasNo: 1177-87-3
Molecular Formula: C24H31FO6
Appearance: White or almost white crystalline powder
Factory Supply Industrial Grade Dexamethasone Acetate 1177-87-3 with Best Price
- Molecular Formula:C24H31FO6
- Molecular Weight:434.505
- Appearance/Colour:White or almost white crystalline powder
- Vapor Pressure:7.75E-16mmHg at 25°C
- Melting Point:238-240 ºC(lit.)
- Refractive Index:87 ° (C=1, Dioxane)
- Boiling Point:579.4 ºC at 760 mmHg
- PKA:12.08±0.70(Predicted)
- Flash Point:304.2 ºC
- PSA:100.90000
- Density:1.3 g/cm3
- LogP:2.46650
Dexamethasone-17-acetate(Cas 1177-87-3) Usage
|
Manufacturing Process |
The preparation of dexamethasone acetate is described in US Patent 3,007,923 as follows. 1.5 cc of dimethylformamide and 1.5 cc of anhydrous hydrofluoric acid are admixed and treated with 480 mg of 9β,11β-epoxy-17αhydroxy-21-acetoxy-16α-methyl-?1,4-pregnadiene-3,20-dione (prepared according to E.P. Oliveto et al, J. Am. Chem. Soc., 80, 44331, 1958). The steroid dissolves in about 15 minutes. The reaction mixture is shaken for two hours at a temperature between 0 and +5°C, and then poured into 75 cc ofwater containing in suspension, 7.5 grams of sodium bicarbonate. The mixture is vacuum filtered, the filter cake washed and then dried at 100°C, yielding 460 mg of crude hexadecadrol contaminated with a small amount of the starting material. A single recrystallization from methylene chloride yields 370 mg of the pure product having a melting point of 170°C and 229°C. The mother liquor yields 62 mg of the starting material, and a remainder constituting a mixture of starting and final materials with little other contamination. |
|
Therapeutic Function |
9-Fluoro-11β,17-dihydroxy-21-acetoxy-16α-methylpregna1,4-diene-3,20-dione |
|
Safety Profile |
Experimental teratogenic and reproductive effects. A steroid. When heated to decomposition it emits toxic fumes of F-. |
|
Purification Methods |
Dexamethasone 21-acetate is purified on neutral Al2O3 using CHCl3 as eluent, the fractions are evaporated, and the residue is recrystallised from CHCl3. It has max at 239nm. [Oliveto et al. J Am Chem Soc 8 0 4431 1958]. [Beilstein 8 IV 3501.] |
|
Brand name |
Decadron (Merck). |
InChI:InChI=1/C24H31FO6/c1-13-9-18-17-6-5-15-10-16(27)7-8-21(15,3)23(17,25)19(28)11-22(18,4)24(13,30)20(29)12-31-14(2)26/h7-8,10,13,17-19,28,30H,5-6,9,11-12H2,1-4H3/t13-,17?,18?,19+,21+,22+,23+,24+/m1/s1
1177-87-3 Relevant articles
C21 steroid 21 site acetylation process
-
Paragraph 0022-0024, (2018/11/22)
The invention discloses a C21 steroid 21...
Ring opening and fluoridation method and device of steroidal epoxy compound
-
Paragraph 0038; 0039; 0041, (2017/07/22)
The invention discloses a method of prep...
Preparation method for dexamethasone sodium phosphate
-
Paragraph 0014, (2016/11/09)
The invention relates to a preparation m...
Preparation technology for dexamethasone sodium phosphate
-
Paragraph 0014, (2016/10/08)
The invention relates to a preparation t...
1177-87-3 Process route
-
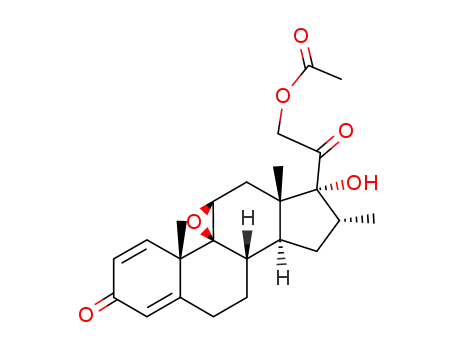
-
2884-51-7
17α,21-dihydroxy-9β,11β-epoxy-16α-methylpregna-1,4-diene-3,20-dione 21-acetate

-
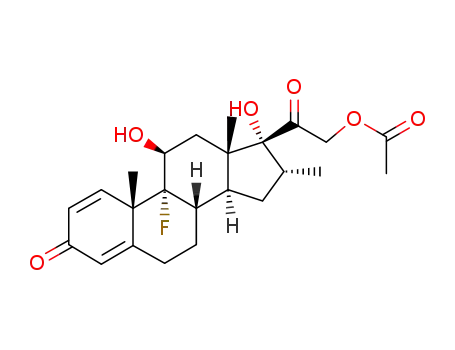
-
1177-87-3
betamethasone
| Conditions | Yield |
|---|---|
|
With
hydrogen fluoride;
In
N,N-dimethyl-formamide;
at -10 ℃;
for 3h;
Temperature;
|
|
|
With
hydrogen fluoride;
In
chloroform;
|
-
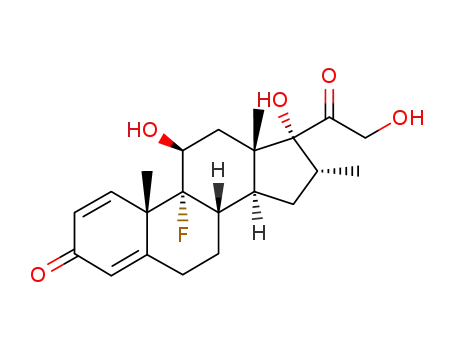
-
50-02-2
dexamethasone

-
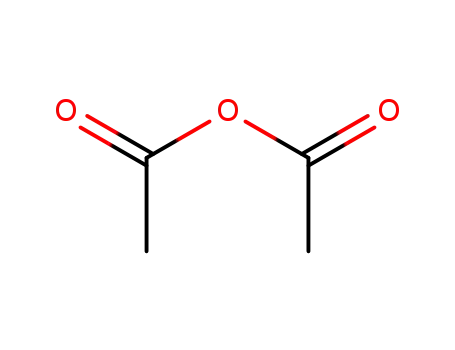
-
108-24-7
acetic anhydride

-

-
1177-87-3
betamethasone
| Conditions | Yield |
|---|---|
|
With
sodium acetate;
In
tetrahydrofuran; acetone;
at 40 ℃;
for 5h;
Inert atmosphere;
|
1177-87-3 Upstream products
-
19784-87-3
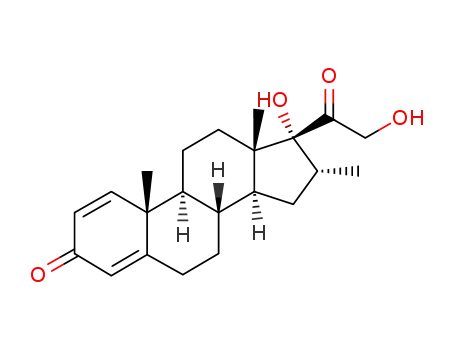
17,21-dihydroxy-16α-methyl-pregna-1,4-diene-3,20-dione
-
115606-30-9
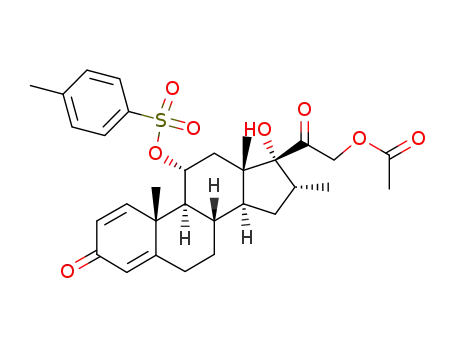
21-acetoxy-17-hydroxy-16α-methyl-11α-(toluene-4-sulfonyloxy)-pregna-1,4-diene-3,20-dione
-
78761-59-8
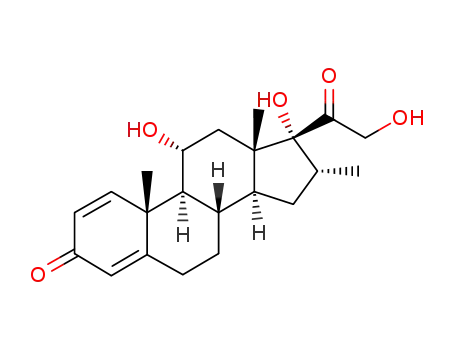
11α,17,21-trihydroxy-16α-methyl-pregna-1,4-diene-3,20-dione
-
6242-16-6
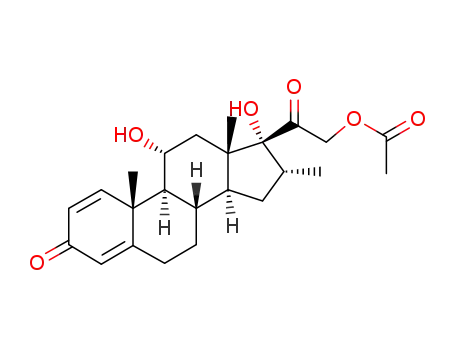
21-acetoxy-11α,17-dihydroxy-16α-methyl-pregna-1,4-diene-3,20-dione
1177-87-3 Downstream products
-
50-02-2

dexamethasone
-
312-93-6
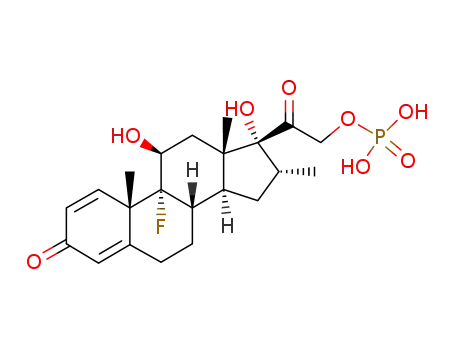
dexamethasone phosphate
-
83880-70-0
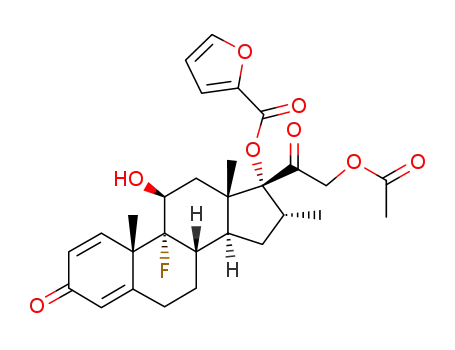
9α-fluoro-16α-methyl-11β,17α,21-trihydroxy-1,4-pregnadiene-3,20-dione 17-(2'-furoate) 21-acetate
Relevant Products
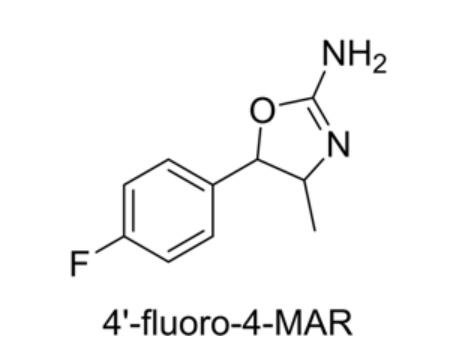
-
para-fluoro Methylaminorex
CAS:1364933-64-1
-
Dolutegravir; DTG; GSK1349572
CAS:1051375-16-6
-
Amiodarone hydrochloride
CAS:19774-82-4