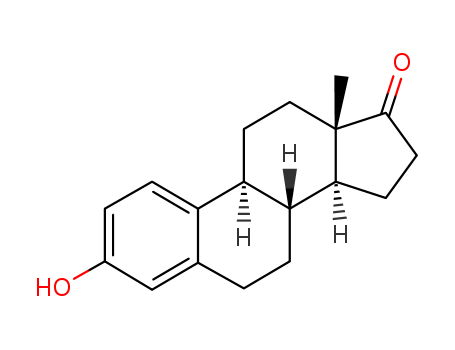
53-16-7
- Product Name:1,3,5(10)-Estratrien-3-ol-17-one
- Molecular Formula:C18H22O2
- Purity:99%
- Molecular Weight:270.371
Product Details;
CasNo: 53-16-7
Molecular Formula: C18H22O2
Appearance: crystalline solid
Quality products make an important contribution to long-term revenue and profitability. Hot sale! Top purity Estrone 53-16-7 in stock
1.What is the 1,3,5(10)-Estratrien-3-ol-17-one ?
Estrone, the major postmenopausal estrogen, binds ERa to induce SNAI2, epithelial-to-mesenchymal transition, and ER+ breast cancer metastasis. Estrone is a weak form of estrogen and exists as estrone sulphate. Estrone is a luteolytic estrogen produced by the corpus luteum. In the follicle, estrone is synthesized from androstenedione by the action of cytochrome P450 aromatase. The photodegradation of the steroid estrone (E1), an endocrine disrupting hormone which is commonly released into aquatic environments.
-
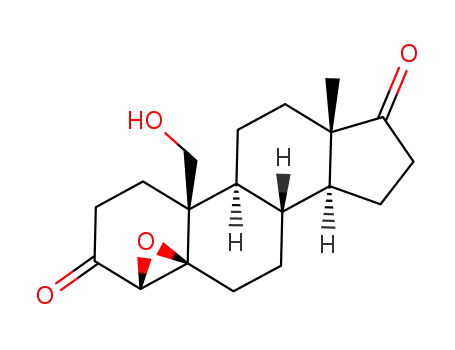
- 53875-00-6
19-hydroxy-4β,5-epoxy-5β-androstane-3,17-dione

-
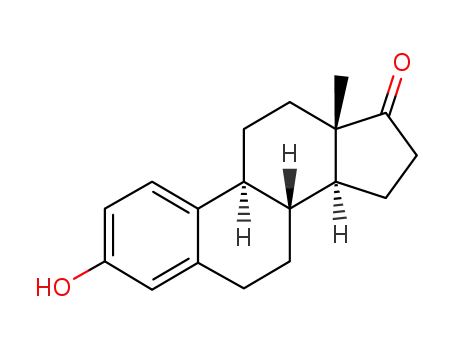
- 53-16-7
Estrone

-
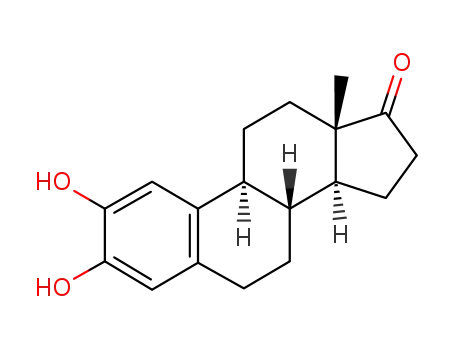
- 362-06-1,4735-38-0
2-hydroxyestrone

-
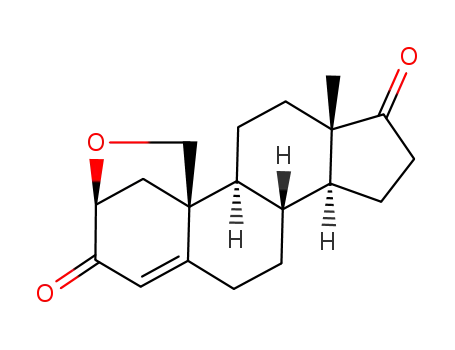
- 124522-67-4
2β,19-epoxyandrost-4-ene-3,17-dione

-
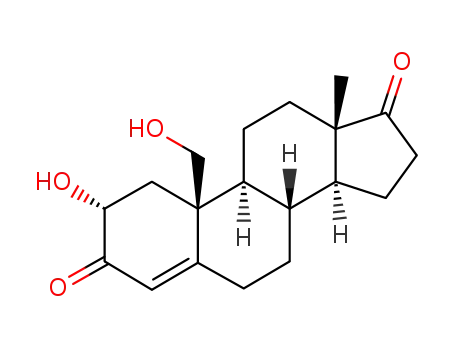
- 53875-01-7
2α,19-dihydroxyandrost-4-ene-3,17-dione
| Conditions | Yield |
|---|---|
|
With sulfuric acid; In dimethyl sulfoxide;
|
8% 21% 25% 16% |
|
With sulfuric acid; In dimethyl sulfoxide; THF/HClO4 also used;
|
8% 21% 16% 25% |
|
With sulfuric acid; In dimethyl sulfoxide; at 120 ℃; for 10h; Yield given; THF/HClO4 also used;
|
8% 16% 24% |
|
With sulfuric acid; In dimethyl sulfoxide; Yields of byproduct given;
|
16% 24% 8% |
-
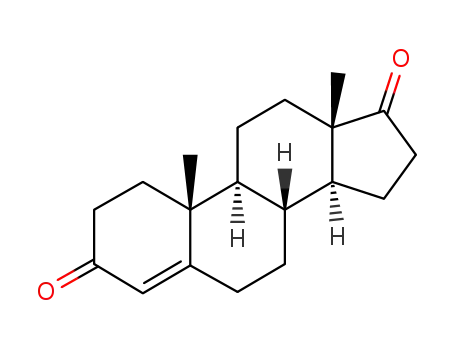
- 63-05-8
Androstenedione

-
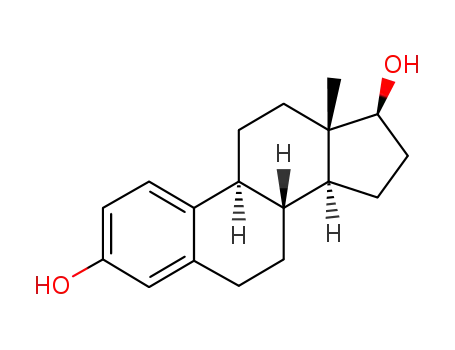
- 50-28-2
estradiol

-

- 53-16-7
Estrone

-
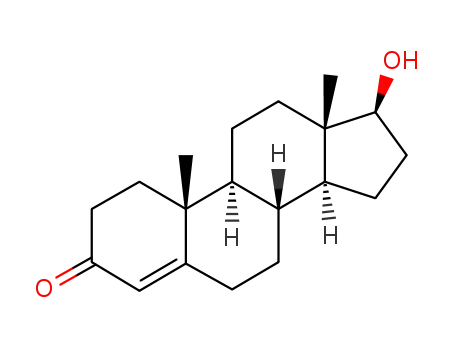
- 58-22-0
testosterone

-
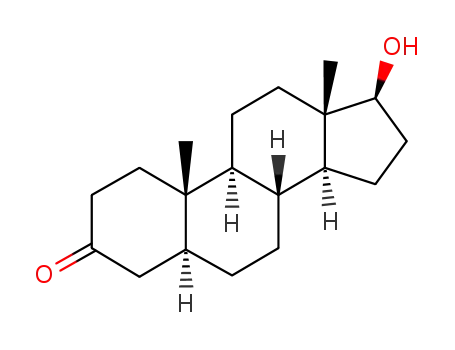
- 521-18-6
Stanolone

-
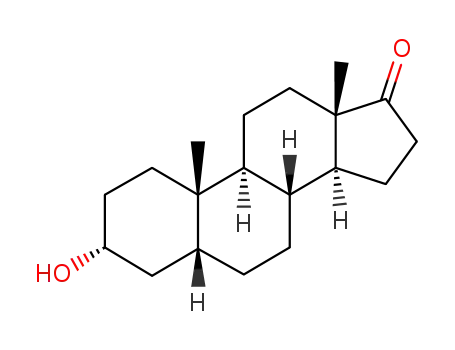
- 53-42-9
Etiocholanolone

-
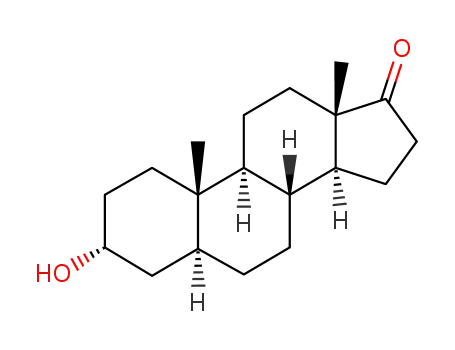
- 53-41-8
cis-androsterone
| Conditions | Yield |
|---|---|
|
With carcinoma; gynecomastia; mammary dysplasia; at 37 ℃; for 1.5h; Product distribution; cofactors under 95percent O2: 5percent CO2, <3H>labeled study;
|
2.What is the CAS number for 1,3,5(10)-Estratrien-3-ol-17-one ?
The CAS number of 1,3,5(10)-Estratrien-3-ol-17-one is 53-16-7.
More information of 1,3,5(10)-Estratrien-3-ol-17-one 53-16-7 are:
|
CAS Number |
53-16-7 |
|
Density |
1.164 g/cm3 |
|
Melting Point |
258-261 ºC |
|
Boiling Point |
445.2 ºC at 760 mmHg |
|
Flash Point |
189.7 ºC |
|
Vapor Pressure |
1.54E-08mmHg at 25°C |
|
Refractive Index |
165 ° (C=1, Dioxane) |
|
HS CODE |
29335995 |
|
PSA |
37.30000 |
|
LogP |
3.81740 |
|
Pka |
pKa 10.77±0.02(H2O)(Approximate) |
3.What are another words for 1,3,5(10)-Estratrien-3-ol-17-one ?
Synonyms for 1,3,5(10)-Estratrien-3-ol-17-one 53-16-7:delta-1,3,5-estratrien-3-beta-ol-17-one;Hormestrin;Oestrin;delta-1,3,5-oestratrien-3-beta-ol-17-one;Estrona [Spanish];CMC_13458;Ovifollin;Wynestron;component of Mal-O-Fem;Cristallovar;Menagen;Estrusol;hydroxyestrones;Ketohydroxyoestrin;.delta.-1,3, 5-Oestratrien-3.beta.-ol-17-one;Folipex;3-Hydroxy-17-keto-estra-1,3,5-triene;Disynformon;3-Hydroxy-oestra-1,3, 5(10)-trien-17-one;Estrone-A;Destrone;Ovex (tablets);Estrol;Follicunodis;
4.What is the molecular formula of 1,3,5(10)-Estratrien-3-ol-17-one?
The chemical formula of 1,3,5(10)-Estratrien-3-ol-17-one is C18H22O2 which containing 18 Carbon atoms,22 Hydrogen atoms and 2 Oxygen atoms,and the molecular weight of 1,3,5(10)-Estratrien-3-ol-17-one is 270.371.
5.What is 1,3,5(10)-Estratrien-3-ol-17-one (53-16-7) used for?
Estrone (E1) has been used:as medium supplement for hormone based degranulation studies of natural killer cells.as an endocrine disrupting compound for screening bacterial biosensor in toxic water.as medium component for monitoring fatty acid synthase (FASN) activity in breast adenocarcinoma cell lines. Serum concentrations of estrone in premenopausal women fluctuate according to the menstrual cycle and becomes the most predominant estrogen in postmenopausal women.The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol. Estrone enhanced gene expression of the osteogenic differentiation marker, Runx2 mRNA (150% above control). The hormone significantly increased cell proliferation (38% above control), nitric oxide production (108% above control), alkaline phosphatase activity (50% above control), in addition to stimulation of extracellular matrix mineralization.
InChI:InChI=1/C18H22O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-16,19H,2,4,6-9H2,1H3/t14?,15-,16?,18+/m1/s1
Relevant articles related to 1,3,5(10)-Estratrien-3-ol-17-one:
|
Article |
Source |
| Direct regio- and stereoselective mono- and polyoxyfunctionalization of estrone derivatives at C(sp3)-H bonds | Journal of Catalysis Volume 415, November 2022, Pages 12-18 |
| A Novel Estrone Degradation Gene Cluster and Catabolic Mechanism in Microbacterium oxydans ML-6 | Applied and Environmental Microbiology, Vol. 89, No. 3, 27 February 2023 |
6.Hot sale 1,3,5(10)-Estratrien-3-ol-17-one with the best price .
Shandong Hanjiang Chemical Co., Ltd. is a quality supplier of 1,3,5(10)-Estratrien-3-ol-17-one. Our main goal is customer satisfaction. Contact us to negotiate the best price for your business on top purity 1,3,5(10)-Estratrien-3-ol-17-one 53-16-7.
Relevant Products
-
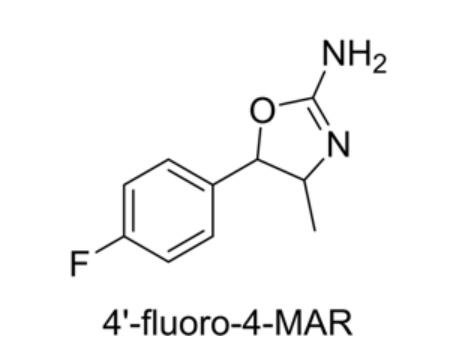
para-fluoro Methylaminorex
CAS:1364933-64-1
-
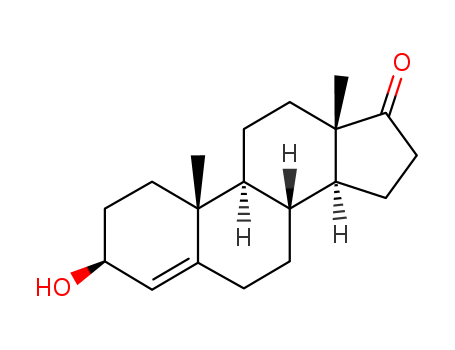
4-Androsten-3b-ol-17-one
CAS:571-44-8
-
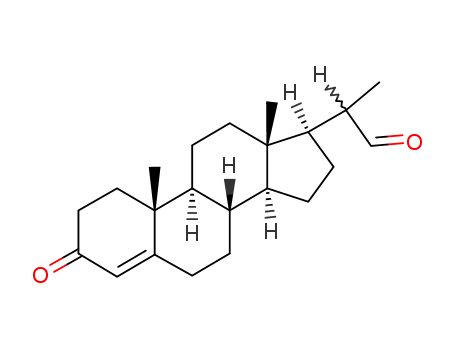
3-Oxopregn-4-ene-20-carbaldehyde
CAS:24254-01-1