106463-17-6
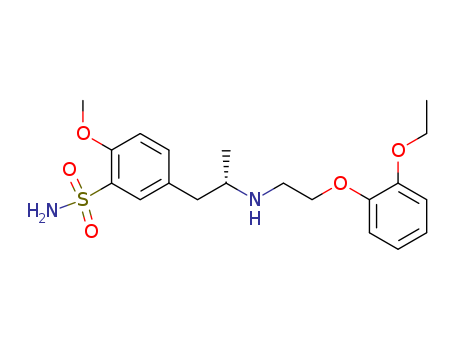
- Product Name:Tamsulosin hydrochloride
- Molecular Formula:C20H29ClN2O5S
- Purity:99%
- Molecular Weight:408.519
Product Details;
CasNo: 106463-17-6
Molecular Formula: C20H29ClN2O5S
Appearance: white to off-white solid
Quality Factory Supply 99% Pure Tamsulosin hydrochloride 106463-17-6 with Efficient Delivery
- Molecular Formula:C20H29ClN2O5S
- Molecular Weight:408.519
- Appearance/Colour:white to off-white solid
- Vapor Pressure:3.79E-14mmHg at 25°C
- Melting Point:228-230 °C
- Boiling Point:595.5 °C at 760 mmHg
- Flash Point:313.9 °C
- PSA:108.26000
- LogP:4.51290
Tamsulosin hydrochloride(Cas 106463-17-6) Usage
|
Therapeutic Function |
Antihypertensive |
|
Hazard |
Moderately toxic by ingestion. |
|
Biological Activity |
Selective α 1A -adrenoceptor antagonist (pK i values are 9.97, 9.64 and 8.86 for α 1A , α 1B and α 1D subtypes respectively). Decreases resting maximal urethral pressure with negligable effect on mean arterial blood pressure in vivo . |
|
Biochem/physiol Actions |
Tamsulosin is an α1A/1D-adrenoceptor antagonist used as a treatment of benign prostatic hypertrophy (BPH). Its activity as an ? blocker also affects the iris, and has led to complications during cataract surgery, a condition called "floppy iris" syndrome. |
|
General Description |
Tamsulosin hydrochloride is a subtypeselective a1A and a1D adrenoceptor antagonist, which exists in two enantiomeric forms, of which the R-isomer is the pharmaceutically active component. It is used to reduce urinary obstruction and is also involved in relieving the symptoms associated with symptomatic benign prostatic hyperplasia. |
InChI:InChI=1/C20H28N2O5S.ClH/c1-4-26-17-7-5-6-8-18(17)27-12-11-22-15(2)13-16-9-10-19(25-3)20(14-16)28(21,23)24;/h5-10,14-15,22H,4,11-13H2,1-3H3,(H2,21,23,24);1H
106463-17-6 Relevant articles
Continuous-Flow Synthesis of (R)-Tamsulosin Utilizing Sequential Heterogeneous Catalysis
Ishitani, Haruro,Kobayashi, Shū,Laroche, Benjamin,Nishizawa, Ken,Saito, Yuki
, (2022/02/16)
We describe the continuous-flow synthesi...
METHOD FOR PRODUCING OPTICALLY ACTIVE AMINE
-
Page/Page column 12, (2010/04/25)
The present invention provides a method ...
Process for the resolution of racemic (R,S) -5-(2-(2-(2- ethoxyphenoxy) ethylamino)propyl)-2-methoxybenzene sulfonamide (tamsulosin), its novel R and S isomers and their salts and processes for their preparation
-
Page/Page column 8; 9, (2010/10/20)
An improved process is described to reso...
Process for the separation of R(-)-and S(+)-5-[2-[[2-(2-ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide
-
Page 6; Sheet 3, (2010/02/10)
The process for separating the R(?)- and...
106463-17-6 Process route
-
-
852619-17-1
C20H26N2O5S

-
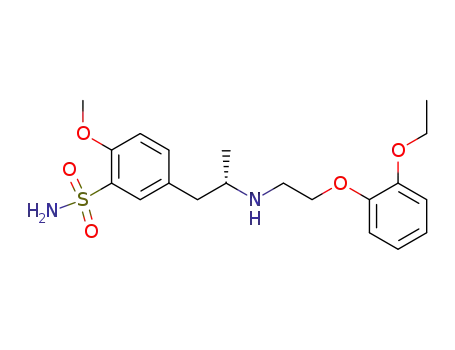
-
106463-17-6,106138-88-9
(+)-Tamsulosin

-
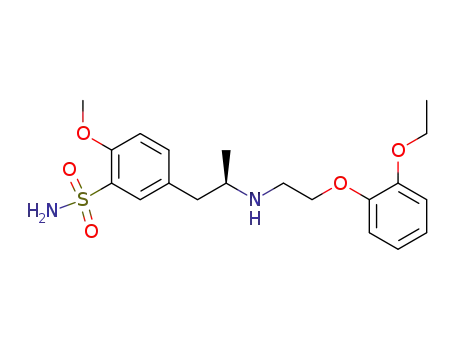
-
106133-20-4,106138-88-9
(R)-tamsulosin
| Conditions | Yield |
|---|---|
|
C20H26N2O5S;
(S)-chloro[(1,2,3,4-η)-pentamethyl-2,4-cyclopentadien-1-yl] [N-(2-methoxy-3-dibenzofuranyl)-2-pyrrolidinecarboxamidato-κN1,κN2]iridium;
In
acetonitrile;
at -3 - 3 ℃;
for 0.5h;
With
formic acid; triethylamine;
In
acetonitrile;
at -3 - 20 ℃;
|
41.4 % ee |
-
![(R/S)-5-[2-[[2-(2-ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide D-10-camphorsulfonic acid salt](/upload/2025/4\f52ce830-e272-4677-aae2-46d94dd3c7b1.png)
-
(R/S)-5-[2-[[2-(2-ethoxyphenoxy)ethyl]amino]propyl]-2-methoxybenzenesulfonamide D-10-camphorsulfonic acid salt

-

-
106463-17-6,106138-88-9
(+)-Tamsulosin

-

-
106133-20-4,106138-88-9
(R)-tamsulosin
| Conditions | Yield |
|---|---|
|
With
sodium hydrogencarbonate;
In
dichloromethane; water;
|
106463-17-6 Upstream products
-
882494-06-6
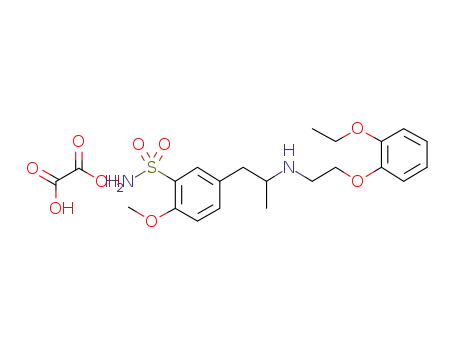
oxalate salt of tamsulosin
-
35193-63-6
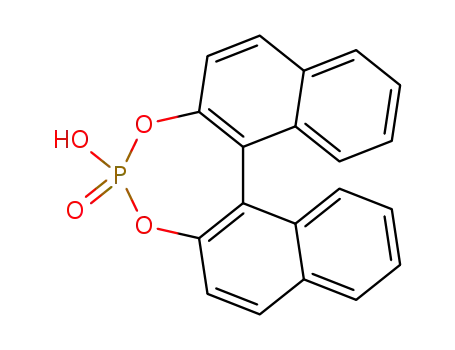
(S)-1,1'-binaphthyl-2,2'-diyl hydrogen phosphate
-
852619-17-1

C20H26N2O5S
-
3250-73-5
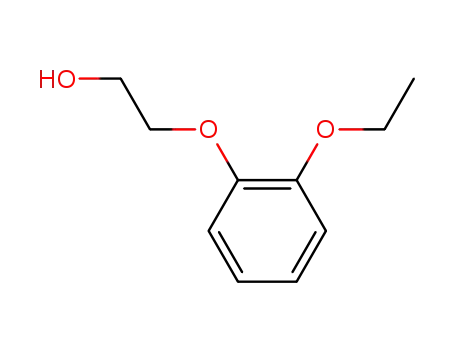
2-(2-ethoxyphenoxy)ethanol
106463-17-6 Downstream products
-
521300-99-2
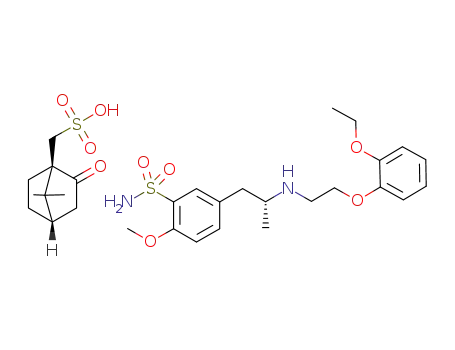
(R)-tamsarocin (-)-camphor-10-sulfonate
-
80223-99-0
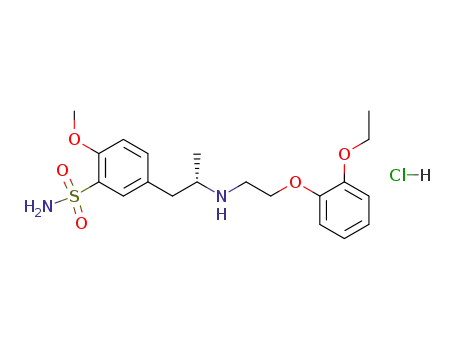
(+)-Tamsulosin hydrochloride
Relevant Products
-
Chlorimuron
CAS:99283-00-8
-
Montelukast sodium
CAS:151767-02-1
-
Aripiprazole
CAS:129722-12-9