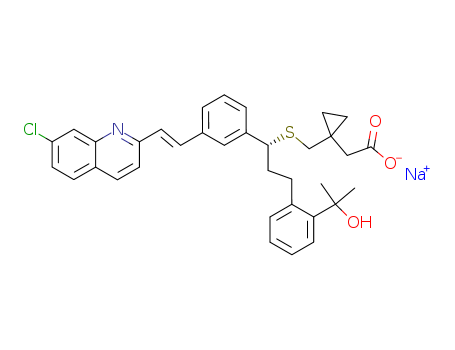
151767-02-1
- Product Name:Montelukast sodium
- Molecular Formula:C35H35ClNNaO3S
- Purity:99%
- Molecular Weight:608.177
Product Details;
CasNo: 151767-02-1
Molecular Formula: C35H35ClNNaO3S
Appearance: White to off-white crystalline powder
China cas 151767-02-1 factory wholesale Montelukast sodium at affordable price
- Molecular Formula:C35H35ClNNaO3S
- Molecular Weight:608.177
- Appearance/Colour:White to off-white crystalline powder
- Melting Point:115 °C(dec.)
- Boiling Point:750.5 °C at 760 mmHg
- Flash Point:407.7 °C
Montelukast sodium(Cas 151767-02-1) Usage
|
Pharmacological effect |
Cysteinyl leukotrienes (LTC4, LTD4, LTE4) is a eicosane-type substance released by various kinds of cells including mast cells and eosinophils with strong inflammatory effect. These important asthma pre-inflammatory mediators can bind to the Cysteinyl leukotriene receptors (CysLT) identified in the human airways, resulting in a variety of airway responses including bronchoconstriction, mucus secretion, increased vascular permeability, and eosinophil accumulation. Montelukast sodium is an orally active selective leukotriene receptor antagonist that can specifically inhibit the cysteinyl leukotriene receptor. It was successfully developed by the Merck Company (German) and had entered into market in Canada, Finland, and Mexican in 1997. It is suitable for the prevention and long-term treatment of adults and children asthma, including the prevention of daytime and nighttime asthma symptoms, the treatment of asthma patients who are aspirin-sensitive and prevention of exercise-induced bronchial contraction, it can also be used to relieve the seasonal allergic rhinitis symptoms of 15 year-old or over 15 year-old patients whose symptoms are invalid and intolerant to other treatment. Montelukast is a selective leukotriene receptor antagonist and has been approved for the oral administration treatment of asthma and allergic rhinitis. It is also a potent oral preparation that can significantly improve the inflammatory indicators. Biological determination of biochemistry and pharmacology has showed that montelukast sodium has a high affinity and selectivity to the CysLT1 receptors (compared with other kinds of pharmacologically important airway receptors such as prostanoid, cholinergic and β-adrenergic receptors). Montelukast can effectively suppress the physiological effects caused by the binding between LTC4, LTD4 and LTE4 receptor and CysLT1 receptor without any receptor agonistic activity. There is the secondary type of cysteinyl leukotriene receptor (CysLT2) presented in the lungs cysteinyl leukotriene receptor but may be limited to the blood vessels. So far, researchers haven’t cloned two receptors so the situation of CysLT receptor is illustrated through binding assay and pharmacological analysis. It has been now thought that montelukast does not antagonize CysLT2 receptors. The above information is edited by the lookchem of Dai Xiongfeng. |
|
Manufacturing Process |
Crotonaldehyde (3.23 mol) in 100 mL of 2-butanol was added dropwise to a refluxing solution of 4-chloroaniline (3.23 mol), p-chloranil (3.23 mol) and HCl conc. (808 mL) in 5.4 L of 2-butanol. After 2 hours of heating 2.7 L of solvent was removed under vacuum at 60°C. Then 2 L of toluene was added to the reaction mixture followed by removal of 2.5-3 L of solvent until a very pasty solid formed. THF (2 L) was added and the mixture heated 30 min after which it was cooled to 0°C. The solid was collected and washed with THF until pure by tlc. The solid was then dissolved in aq. K2CO3/EtOAc and the organic phase separated. The aqueous phase was extracted with EtOAc and the organic phases combined, dried over MgSO4 and the solvent removed. The product was crystallized in the minimum amount of EtOAc to give 328.08 g (57%) of 4-chloro-2-methylquinolin.4-Chloro-2-methylquinalin was converted into 3-(2-(7-chloro)-2- quinolinyl)ethenyl)benzaldehyde. Reaction was carried out according to a method described in U.S. Pat. No. 4,851,409To a degassed suspension of 3-(2-(7-chloro-2-quinolinyl)ethenyl)benzaldehyde (0.34 mol) in toluene (700 mL) at 0°C was added 1.0 M vinylmagnesium bromide in toluene/THF (370 mL). After stirring for 1 hour at 0°C, the reaction was quenched by the addition of saturated NH4Cl solution (150 ml), followed by H2O (500 mL) and HOAc (50 mL). The product was extracted with EtOAc and the two-phase system was filtered through celite to remove an insoluble precipitate. The aqueous phase was then re-extracted with EtOAc (100 mL) and the combined organic layer was washed with H2O, followed by brine. The solution was dried (MgSO4), and evaporated to give a dark yellow residue which was purified by flash chromatography (EtOAc:hexane 1:5, then 1:3). The product was filtered from the column fractions to give a solid of 1- (3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-2-propen-1-ol (melting point = 110-112°C). The filtrate was concentrated and the resulting residue was recrystallized from EtOAc/hexane 1:4 to give a second crop of 15.1 g.A degassed suspension of 1-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-2-propen-1-ol (46.6 mmol), n-Bu4NCl (93 mmol), LiOAcH2O (115 mmol), LiCl (93 mmol), Pd(OAc)2 (1.4 mmol) and methyl 2-(2-iodophenyl)propanoate in DMF (90 mL) was stirred for 2 hours at 100°C. The dark red solution was then cooled to 0°C and poured into saturated NaHCO3 solution (500 mL). The product was extracted with EtOAc and the organic layer was washed with H2O followed by brine. The solvent was removed under vacuum and the residue was purified by flash chromatography (EtOAc:hexane 1:10, 1:5 and 3:10) to give a pale yellow foam of ethyl 2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl) phenyl)-3-hydroxy-propyl)benzoate (18.9 g).A mixture of anhydrous CeCl3 (164 mmol) in THF (500 mL) was refluxed overnight using a Dean Stark trap filled with activated molecular sieves. Methyl magnesium chloride (3.0 Molar solution in THF, 790 mmol) was added dropwise over 30 min to the CeCl3 slurry at 0°C. After stirring 2 hours, the mixture was cooled to -5°C and a toluene (600 mL) solution of the ethyl 2- (3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-hydroxy-propyl)benzoate (152 mmol) was added dropwise over 1 hour. The reaction mixture was stirred another hour before the addition of 2 M HOAc (600 mL) and toluene (600 mL). The organic layer was washed with saturated aq. NaHCO3 and with brine. Concentration in vacuo and purification of the residue by flash chromatography (30% EtOAc in toluene) gave 63.48 g (91%) of the 2-(2- (3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-hydroxypropyl)phenyl)-2- propanol.To a solution of BH3THF complex (1 M in THF, 262 mL) was added diethyl 1,1- cyclopropanedicarboxylate (134 mmol) at 25°C under N2. The solution was heated at reflux for 6 hours, cooled to r.t., and MeOH (300 mL) was cautiously added. The solution was stirred for 1 hour and then concentrated to an oil. The crude 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3- hydroxypropyl)phenyl)-2-propanol was dissolved in CH2Cl2 (234 mL) and SOCl2 (15.9 g, 134 mmol) was added dropwise over a period of 15 min at 25°C. After stirring for another 15 min, the mixture was washed with aqueous NaHCO3. The organic extract was dried over Na2SO4, filtered and concentrated to give quantitatively the 1,1-cyclopropanedimethanol cyclic sulfite.To a solution of the 1,1-cyclopropanedimethanol cyclic sulfite (99 mmol) in DMF (83 mL) was added NaCN (199 mmol). The mixture was heated to 90°C for 20 hours. Upon cooling, EtOAc (400 mL) was added and the solution was washed with saturated NaHCO3 solution (55 mL), H2O (4 times 55 mL), saturated NaCl solution and dried over Na2SO4. The solution was concentrated to give 7.1 g (65%) of 1-(hydroxymethyl)cyclopropaneacetonitrile.To a solution of 1-(hydroxymethyl)cyclopropaneacetonitrile (42 g, 378 mmol) in dry CH2Cl2 (450 mL) at -30°C was added Et3N (741 mmol) followed by CH3SO2Cl (562 mmol) dropwise. The mixture was warmed to 25°C, washed with NaHCO3, dried over Na2SO4 and concentrated in vacuo to give the corresponding mesylate. The mesylate was then dissolved in DMF (450 mL) and cooled to 0°C. Potassium thioacetate (55.4 g, 485 mmol) was added, and the mixture was stirred at 25°C for 18 hours. EtOAc (1.5 L) was added, the solution was washed with NaHCO3, dried over Na2SO4 and concentrated in vacuo to give 45 g (70%) of 1-(acetythiomethyl)cyclopropaneacetonitrile.To a solution of the 1-(acetythiomethyl)cyclopropaneacetonitrile (266 mmol) in MeOH (1.36 L) was added H2O (84 mL) and conc. H2SO4(168 mL). The mixture was heated to reflux for 20 hours, cooled to 25°C, H2O (1 L) was added and the product was extracted with CH2Cl2. The organic extract was washed with H2O and dried over Na2SO4. Concentration of the organic solution gave 36 g (93%) of the methyl 1-(thiomethyl)cyclopropaneacetate.To a solution of 2-(2-(3(S)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3- hydroxypropyl)phenyl)-2-propanol in THF was dissolved in THF (1 mL) and DMF (1 mL) at -40°C was added diisopropylethylamine (2.2 mmol) and then methanesulfonyl chloride (2.2 mmol). The mixture was stirred 2 hours with slow warming to -30°C. The methyl 1-(thiomethyl)cyclopropaneacetate (2.3 mmol) was added to the cloudy reaction mixture followed by dropwise addition of potassium tert-butoxide/THF solution (4.4 mmol). The reaction mixture was stirred at -30°C for 3.5 hours before quenching it with 25% aq NH4OAc. Extraction with EtOAc, washing the organic layer with brine and evaporation of the solvents left a residue that was purified by flash chromatography (5%-10% EtOAc in toluene) giving 658 mg (53%) of methyl 1-((((R)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2- propyl)phenyl)propyl)thio)methyl)cyclopropaneacetate.Following the hydrolysis the methyl 1-((((R)-(3-(2-(7-chloro-2-quinolinyl) ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)phenyl)propyl)thio)methyl) cyclopropaneacetate with NaOH was obtained the free acid: 4-((1(R)-(3-(2-(7- chloro-2-quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)-phenyl)propyl) thio)methyl)cyclopropaneacetic acid or sodium 1-(((1(R)-(3-(2-(7-chloro-2- quinolinyl)ethenyl)phenyl)-3-(2-(2-hydroxy-2-propyl)phenyl)propyl)thio) methyl) cyclopropaneacetate. |
|
Therapeutic Function |
Anti-asthmatic |
|
Biochem/physiol Actions |
Montelukast sodium hydrate is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma and to relieve symptoms of seasonal allergies. It is a subtype specific CysLT1 receptor antagonist. |
|
Brand name |
Singulair (Merck). |
InChI:InChI=1/C35H36ClNO3S.Na/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29;/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39);/q;+1/p-1/b15-10+;
151767-02-1 Relevant articles
Preparation method of montelukast sodium
-
Paragraph 0054-0074; 0083-0086, (2020/12/15)
The invention discloses a preparation me...
Montelukast sodium intermediate compound
-
Paragraph 0066; 0068, (2020/12/08)
The invention provides a novel monteluka...
Synthesis method of montelukast sodium
-
Paragraph 0020; 0050-0067, (2020/11/23)
The invention belongs to the technical f...
A synthesis process of montelukast sodium (by machine translation)
-
, (2019/04/04)
A synthesis process of montelukast sodiu...
151767-02-1 Process route
-
![1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropaneacetic acid R-(+)-α-methylbenzylamine salt](/upload/2025/4\77dc03eb-ca17-47b5-b04c-4c989ca6f607.png)
-
1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropaneacetic acid R-(+)-α-methylbenzylamine salt

-
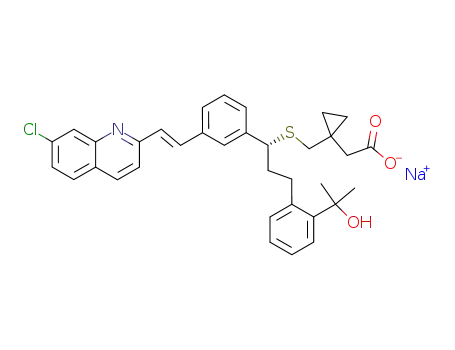
-
151767-02-1
Montelukast sodium
| Conditions | Yield |
|---|---|
|
With
sodium methylate; pyrographite;
In
toluene;
at 25 - 30 ℃;
for 1.5h;
|
93% |
-
-
942303-96-0
montelukast N-methyl-morpholine salt

-

-
151767-02-1
Montelukast sodium
| Conditions | Yield |
|---|---|
|
With
sodium methylate;
In
toluene;
at 25 - 30 ℃;
for 0.5h;
|
90% |
151767-02-1 Upstream products
-
1310-73-2
sodium hydroxide
-
158966-92-8
montelukast
-
1008509-41-8
1-(((1(R)-(3-(2-(7-chloro-2-quinolinyl)ethenyl)-phenyl)-3-(2-(1-hydroxy-1-methylethyl)phenyl)propyl)thio)methyl)-cyclopropaneacetic acid trans-4-aminocyclohexanol salt
-
945934-73-6
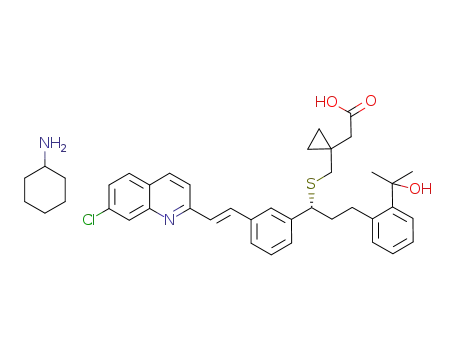
1-[[[(1R)-1-[3-[(1E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropaneacetic acid cyclohexylamine salt
151767-02-1 Downstream products
-
909849-96-3
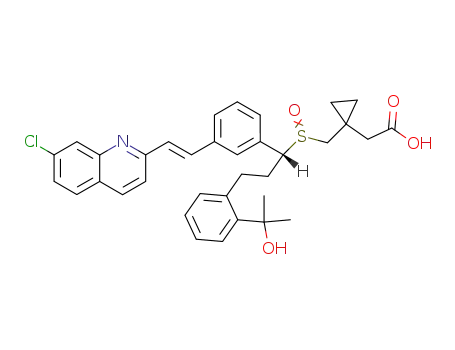
[R,E]-1-[[[1-[3-[2-(7-chloro-2-quinolinyl) ethenyl]-phenyl]-3-[2-(-hydroxy-1-methylethyl) phenyl]propyl]-sulfinyl] methyl] cyclopropaneacetic acid
-
158966-92-8

montelukast
-
1178966-47-6
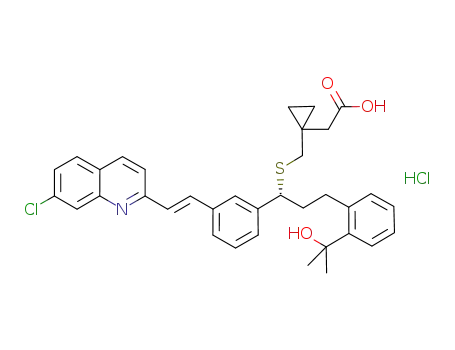
2-(1-((((R)-1-(3-((E)-2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-(2-(2-hydroxypropan-2-yl)phenyl)propyl)sulfanyl)methyl)cyclopropyl) acetic acid hydrochloride
-
780755-34-2
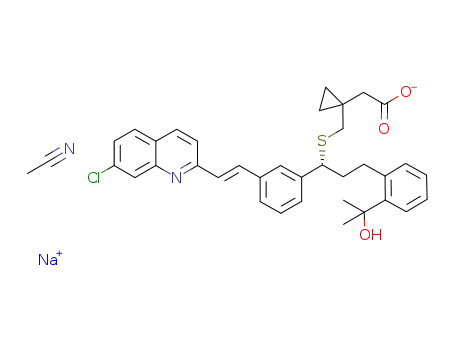
montelukast sodium; acetonitrile monosolvate
Relevant Products
-
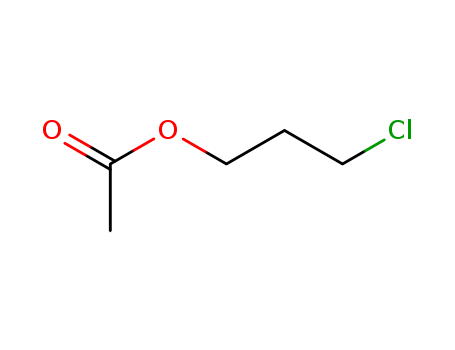
3-Chloropropyl acetate
CAS:628-09-1
-
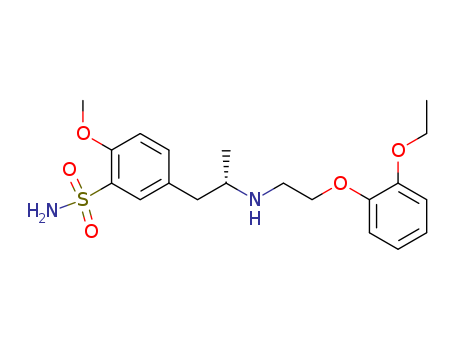
Tamsulosin hydrochloride
CAS:106463-17-6