529-34-0
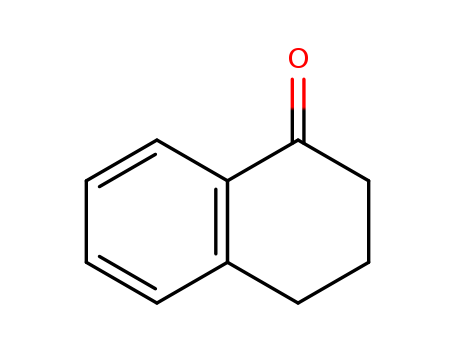
- Product Name:1-Tetralone
- Molecular Formula:C10H10O
- Purity:99%
- Molecular Weight:146.189
Product Details;
CasNo: 529-34-0
Molecular Formula: C10H10O
Appearance: Clear amber to brown oily liquid
Factory supply 1-Tetralone 529-34-0 with sufficient stock and high standard
- Molecular Formula:C10H10O
- Molecular Weight:146.189
- Appearance/Colour:Clear amber to brown oily liquid
- Melting Point:2-7 °C(lit.)
- Refractive Index:1.5685
- Boiling Point:255.8 °C at 760 mmHg
- Flash Point:104.6 °C
- PSA:17.07000
- Density:1.101 g/cm3
- LogP:2.20560
1-Tetralone(Cas 529-34-0) Usage
|
Synthesis Reference(s) |
Tetrahedron Letters, 32, p. 4291, 1991 DOI: 10.1016/S0040-4039(00)92151-8 |
|
Purification Methods |
Check the IR first. Purify α-tetralone by dissolving 20mL in Et2O (200mL), washing with H2O (100mL), 5% aqueous NaOH (100mL), H2O (100mL), 3% aqueous AcOH (100mL), 5% NaHCO3 (100mL) then H2O (100mL) and dry the ethereal layer over MgSO4. Filter, evaporate and fractionate the residue through a 6in Vigreux column (p 11) under reduced pressure to give a colourless oil (~17g) with b 90-91o/0.50.7mm. [Snyder & Werber Org Synth Coll Vol III 798 1955.] It has also been fractionated through a 0.5metre packed column with a heated jacket under reflux using a partial take-off head. It has max 247.5 and 290nm (hexane). The phenylhydrazone has m 83o. The 2,4,6-trinitrophenylhydrazone has m 247.5-248o (from EtOH). [Olson & Bader Org Synth Coll Vol IV 898 1963, Beilstein 7 H 370, 7 III 1416, 7 IV 1015.] |
InChI:InChI=1/C10H10O/c11-10-7-3-5-8-4-1-2-6-9(8)10/h1-2,4,6H,3,5,7H2
529-34-0 Relevant articles
-
Gilmore
, p. 5879 (1951)
-
Impact of metalloporphyrin-based porous coordination polymers on catalytic activities for the oxidation of alkylbenzene
Du, Yan xia,Lü, Xiang-fei,Li, Jun,Mele, Giuseppe,Ni, Wan-kui,Zhao, Yong-guo
, (2020)
Seven metalloporphyrin-based porous coor...
Preparation, characterization, and catalytic application of nano Ag/ZnO in the oxidation of benzylic C-H bonds in sustainable media
Hosseini-Sarvari, Mona,Ataee-Kachouei, Tahereh,Moeini, Fatemeh
, p. 9050 - 9056 (2015)
Nano Ag/ZnO is successfully synthesized ...
Photochemistry of α-fluorocycloalkanones and α-bromo- α- fluorocycloalkanones
Sket,Zupancic,Zupan
, p. 313 - 321 (1989)
Uv irradiation of a cyclohexane solution...
Intramolecular cyclization using palladium-catalyzed arylation toward formyl and nitro groups
Muratake, Hideaki,Nakai, Hiroshi
, p. 2355 - 2358 (1999)
Intramolecular arylation of properly des...
-
Matsuura et al.
, p. 1623 (1962)
-
Ag-Catalyzed ring-opening of tertiary cycloalkanols for C-H functionalization of cyclic aldimines
Wang, Jingjing,Liu, Xue,Wu, Ziyan,Li, Feng,Zhang, Ming-Liang,Mi, Yiman,Wei, Junhao,Zhou, Yao,Liu, Lantao
, p. 1506 - 1509 (2021)
We firstly describe a silver-catalyzed d...
Heterogenization of [Cu(2,2′-bpy)Cl2] and [Cu(1,10-phen)Cl2] on polyoxometalates: New catalysts for the selective oxidation of tetralin
Boltz,Blanc,Laugel,Pale,Louis
, p. 807 - 811 (2011)
Mononuclear Cu(II) bipyridine (1) and ph...
Wet alumina supported chromium(VI) oxide: Selective oxidation of alcohols in solventless system
Varma, Rajender S.,Saini, Rajesh K.
, p. 1481 - 1482 (1998)
A simple and selective method for the ox...
Chelating bis-N-heterocyclic carbene complexes of iron(II) containing bipyridyl ligands as catalyst precursors for oxidation of alcohols
Pinto, Mara F.,Cardoso, Bernardo De P.,Barroso, Sónia,Martins, Ana M.,Royo, Beatriz
, p. 13541 - 13546 (2016)
Chelating bis-N-heterocyclic carbene (bi...
-
Boocock,Waight
, p. 258 (1968)
-
A traceless perfluorooctylsulfonyl tag for deoxygenation of phenols under microwave irradiation
Zhang, Wei,Nagashima, Tadamichi,Lu, Yimin,Chen, Christine Hiu-Tung
, p. 4611 - 4613 (2004)
The perfluorooctylsulfonyl group is intr...
HCl-Catalyzed Aerobic Oxidation of Alkylarenes to Carbonyls
Niu, Kaikai,Shi, Xiaodi,Ding, Ling,Liu, Yuxiu,Song, Hongjian,Wang, Qingmin
, (2021/12/13)
The construction of C?O bonds through C?...
Selective Aerobic Oxidation of Csp3-H Bonds Catalyzed by Yeast-Derived Nitrogen, Phosphorus, and Oxygen Codoped Carbon Materials
Ju, Zhao-Yang,Song, Li-Na,Chong, Ming-Ben,Cheng, Dang-Guo,Hou, Yang,Zhang, Xi-Ming,Zhang, Qing-Hua,Ren, Lan-Hui
supporting information, p. 3978 - 3988 (2022/03/16)
Nitrogen, phosphorus, and oxygen codoped...
Efficient and selective oxidation of hydrocarbons with tert-butyl hydroperoxide catalyzed by oxidovanadium(IV) unsymmetrical Schiff base complex supported on γ-Fe2O3 magnetic nanoparticles
Samani, Mahnaz,Ardakani, Mehdi Hatefi,Sabet, Mohammad
, p. 1481 - 1494 (2022/01/22)
The catalytic activity of an oxidovanadi...
Two transition-metal-modified Nb/W mixed-addendum polyoxometalates for visible-light-mediated aerobic benzylic C–H oxidations
Chen, Xuenian,Gao, Fan,Li, Na,Li, Shujun,Ma, Yubin,Xiao, Wanru,Yu, Bing
supporting information, (2022/03/27)
The visible-light-induced selective oxid...
529-34-0 Process route
-
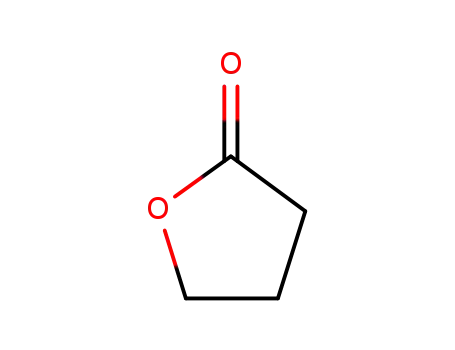
-
96-48-0
4-butanolide

-
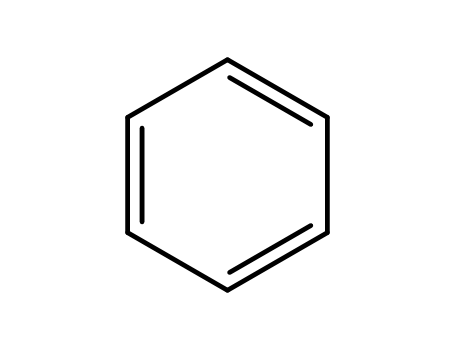
-
71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-
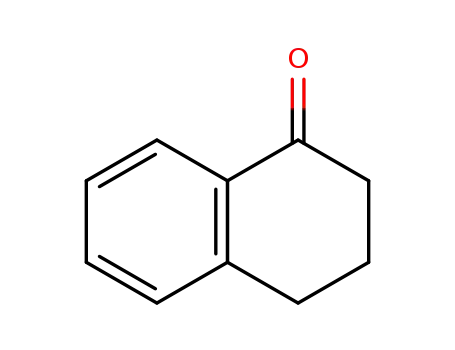
-
529-34-0
3,4-dihydronaphthalene-1(2H)-one

-
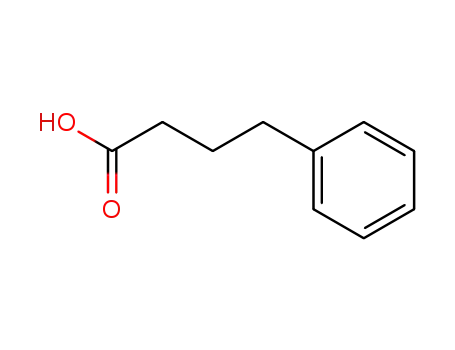
-
1821-12-1
4-Phenylbutyric acid
| Conditions | Yield |
|---|---|
|
With
aluminium trichloride;
|
-
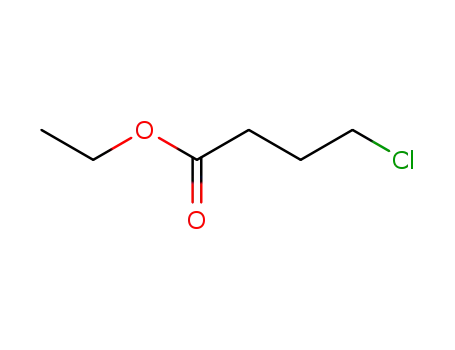
-
3153-36-4
4-chloro-butyric acid ethyl ester

-

-
71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-
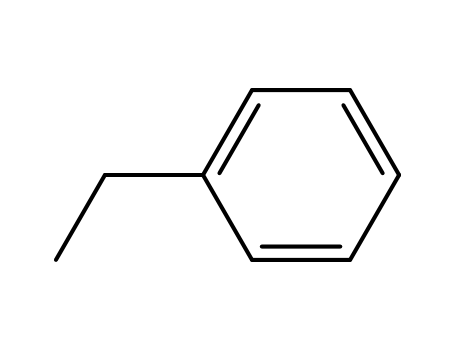
-
100-41-4,27536-89-6
ethylbenzene

-

-
529-34-0
3,4-dihydronaphthalene-1(2H)-one

-

-
1821-12-1
4-Phenylbutyric acid
| Conditions | Yield |
|---|---|
|
|
529-34-0 Upstream products
-
110-86-1
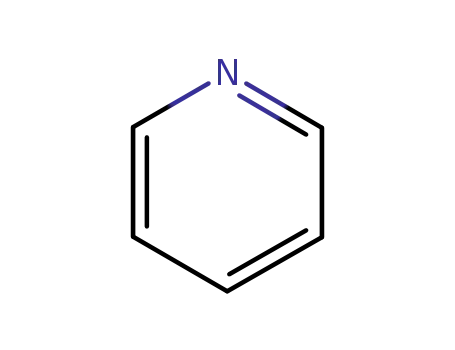
pyridine
-
64245-04-1
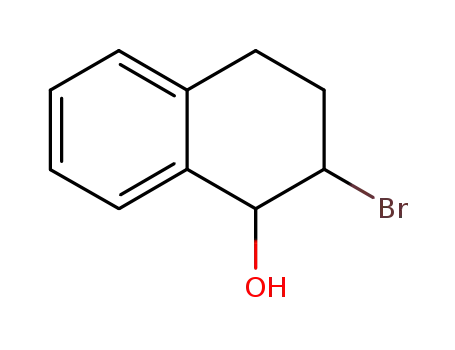
2-bromo-1-hydroxy-1,2,3,4-tetrahydronaphthalene
-
96-48-0

4-butanolide
-
71-43-2

benzene
529-34-0 Downstream products
-
7508-21-6
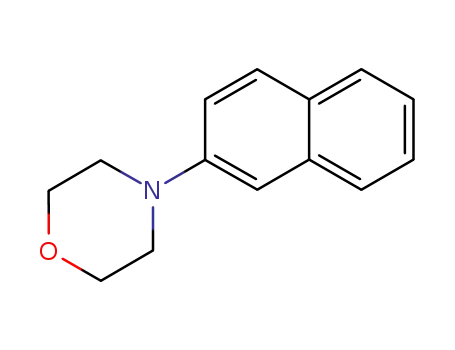
N-(2-naphthyl)morpholine
-
6322-29-8
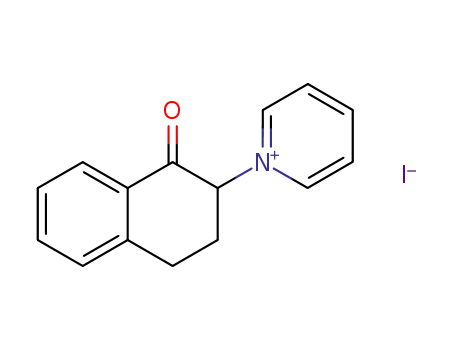
1-(1-oxo-1,2,3,4-tetrahydro-[2]naphthyl)-pyridinium; iodide
-
54752-27-1
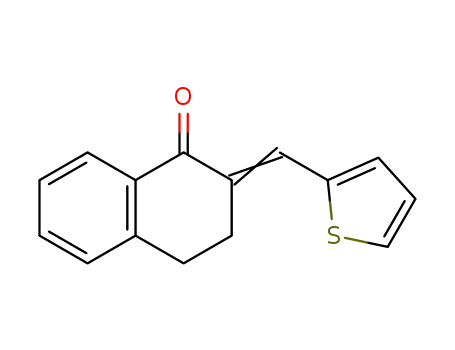
2-(Furan-2′′-yl)methylene-3,4-dihydro-2H-naphthalen-1-one
-
17216-08-9
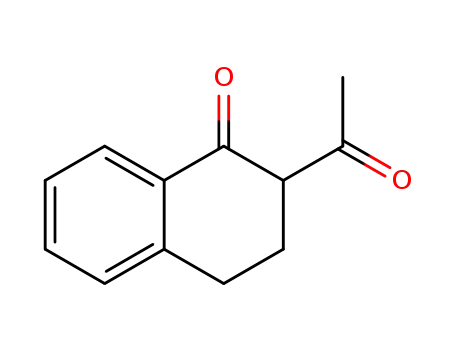
2-acetyl-1-tetralone
Relevant Products
-
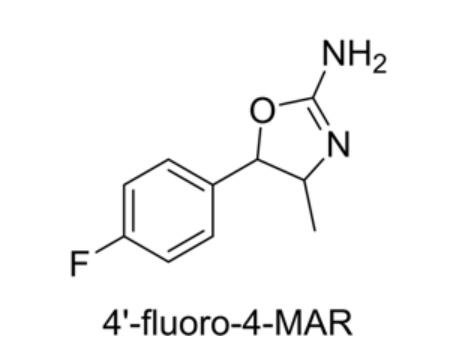
para-fluoro Methylaminorex
CAS:1364933-64-1
-
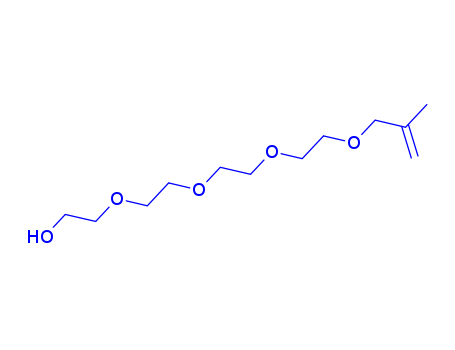
Polyethylene glycol monomethallyl ether
CAS:31497-33-3
-
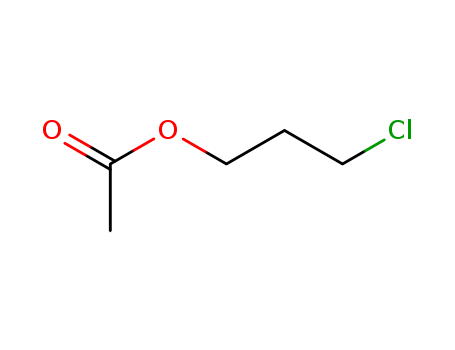
3-Chloropropyl acetate
CAS:628-09-1