104-01-8
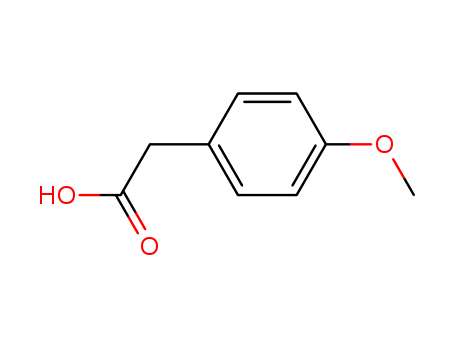
- Product Name:4-Methoxyphenylacetic acid
- Molecular Formula:C9H10O3
- Purity:99%
- Molecular Weight:166.177
Product Details;
CasNo: 104-01-8
Molecular Formula: C9H10O3
Appearance: white to light yellow crystalline powder
Factory Export Top Purity 4-Methoxyphenylacetic acid 104-01-8 In Stock
- Molecular Formula:C9H10O3
- Molecular Weight:166.177
- Appearance/Colour:white to light yellow crystalline powder
- Vapor Pressure:0.143mmHg at 25°C
- Melting Point:84-86 °C(lit.)
- Refractive Index:1.502
- Boiling Point:306 °C at 760 mmHg
- PKA:pK1:4.358 (25°C)
- Flash Point:124.3 °C
- PSA:46.53000
- Density:1.179 g/cm3
- LogP:1.32230
4-Methoxyphenylacetic acid(Cas 104-01-8) Usage
|
Reference |
Chen, Yan Ping, Y. M. Chen, and M. Tang. "Solubilities of cinnamic acid, phenoxyacetic acid and 4-methoxyphenylacetic acid in supercritical carbon dioxide." Fluid Phase Equilibria 275.1(2008):33-38. |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 51, p. 4354, 1986 DOI: 10.1021/jo00373a005Tetrahedron Letters, 35, p. 133, 1994 DOI: 10.1016/0040-4039(94)88182-0 |
|
Reactivity Profile |
Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 4-Methoxyphenylacetic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. |
|
Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
Fire Hazard |
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
|
Flammability and Explosibility |
Notclassified |
|
Safety Profile |
Moderately toxic by ingestion and intraperitoneal routes. Questionable carcinogen with experimental neoplastigenic data. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Purification Methods |
Crystallise the acid from EtOH/water, EtOAc/pet ether (m 87o) or *C6H6/pet ether (m 84-86o). [Beilstein 10 III 431, 10 IV 544.] The acid chloride [4693-91-8] has M 184.6, b 143o/10mm, d 254 |
|
Definition |
ChEBI: A monocarboxylic acid that is phenylacetic acid carrying a 4-methoxy substituent. It is used as an intermediate for pharmaceuticals and other organic synthesis. It has been found to inhibit the germination of cress and lettuce seeds. |
|
General Description |
Pale yellow or off white colored flakes. Severely irritates skin and eyes. May be toxic by ingestion. |
InChI:InChI=1/C8H8O3/c1-10-7-2-4-8(5-3-7)11-6-9/h2-6H,1H3
104-01-8 Relevant articles
Wolff rearrangement of α-diazoketones using in situ generated silver nanoclusters as electron mediators
Sudrik, Surendra G.,Sharma, Jadab,Chavan, Vilas B.,Chaki, Nirmalya K.,Sonawane, Harikisan R.,Vijayamohanan, Kunjukrishna P.
, p. 1089 - 1092 (2006)
We report Wolff rearrangement of α-diazo...
Carboxylation of benzylic and aliphatic C-H bonds with CO2 induced by light/ketone/nickel
Ishida, Naoki,Masuda, Yusuke,Imamura, Yuuya,Yamazaki, Katsushi,Murakami, Masahiro
, p. 19611 - 19615 (2019)
A photoinduced carboxylation reaction of...
-
Greene,F.D. et al.
, p. 3461 - 3468 (1961)
-
-
Kindler,Brandt,Gehlhaar
, p. 209,212 Anm. (1934)
-
Practical and green synthesis of combretastatin A-4 and its prodrug CA4P using renewable biomass-based starting materials
Chen, Yu,Zou, Yong,Sun, Hong-Yi,Liu, Xian-Ke,Xiao, Chun-Fen,Sun, Jie,He, Shu-Jie,Li, Jun
, p. 217 - 222 (2011)
A practical and green protocol for the s...
AlCl3·6H2O/KI/CH3CN/H 2O: An efficient and versatile system for chemoselective C-O bond cleavage and formation of halides and carbonyl compounds from alcohols in hydrated media
Gogoi, Pranjal,Konwar, Dilip,Sharma, Saikat Das,Gogoi, Prodip Kumar
, p. 1259 - 1264 (2006)
AlCl3·6H2O/KI/CH3CN/H 2O, an efficient a...
Pd(OH)2/C, a Practical and Efficient Catalyst for the Carboxylation of Benzylic Bromides with Carbon Monoxide
Wakuluk-Machado, Anne-Marie,Dewez, Damien F.,Baguia, Hajar,Imbratta, Miguel,Echeverria, Pierre-Georges,Evano, Gwilherm
, p. 713 - 723 (2020)
A simple, efficient, cheap, and broadly ...
Silver nanocluster redox-couple-promoted nonclassical electron transfer: An efficient electrochemical wolff rearrangement of α-diazoketones
Sudrik, Surendra G.,Chaki, Nirmalya K.,Chavan, Vilas B.,Chavan, Sambhaji P.,Chavan, Subhash P.,Sonawane, Harikisan R.,Vijayamohanan
, p. 859 - 864 (2006)
In this work we report the unique electr...
The effect of an alkoxy group on the kinetic and thermodynamic acidity of benzene and toluene
Schlosser, Manfred,Maccaroni, Paola,Marzi, Elena
, p. 2763 - 2770 (1998)
2-, 3- and 4-Methoxytoluene can be selec...
Copper-catalyzed oxidation of deoxybenzoins to benzils under aerobic conditions
Cacchi, Sandro,Fabrizi, Giancarlo,Goggiamani, Antonella,Iazzetti, Antonia,Verdiglione, Rosanna
, p. 1701 - 1707 (2013)
A novel copper-catalyzed approach to ben...
HETEROCYCLIC COMPOUND, APPLICATION THEREOF, AND COMPOSITION CONTAINING SAME
-
, (2022/03/07)
A heterocyclic compound represented by f...
Desulfonylative Electrocarboxylation with Carbon Dioxide
Zhong, Jun-Song,Yang, Zi-Xin,Ding, Cheng-Lin,Huang, Ya-Feng,Zhao, Yi,Yan, Hong,Ye, Ke-Yin
supporting information, p. 16162 - 16170 (2021/09/02)
Electrocarboxylation of organic halides ...
Alkali-modified heterogeneous Pd-catalyzed synthesis of acids, amides and esters from aryl halides using formic acid as the CO precursor
Fapojuwo, Dele Peter,Maqunga, Nomathamsanqa Prudence,Meijboom, Reinout,Mogudi, Batsile M.,Molokoane, Pule Petrus,Onisuru, Oluwatayo Racheal,Oseghale, Charles O.
, p. 26937 - 26948 (2021/08/17)
To establish an environmentally friendly...
BF3·OEt2-promoted tandem Meinwald rearrangement and nucleophilic substitution of oxiranecarbonitriles
Xu, Chuangchuang,Xu, Jiaxi
, p. 127 - 134 (2019/12/26)
Tandem Meinwald rearrangement and nucleo...
104-01-8 Process route
-
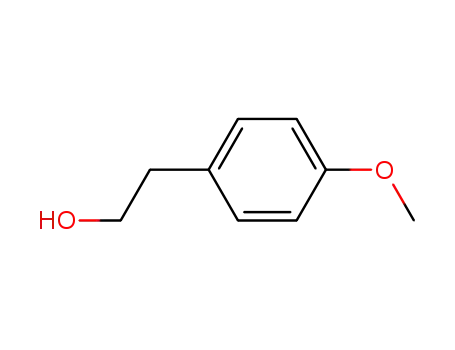
-
702-23-8
2-(4-Methoxyphenyl)ethanol

-
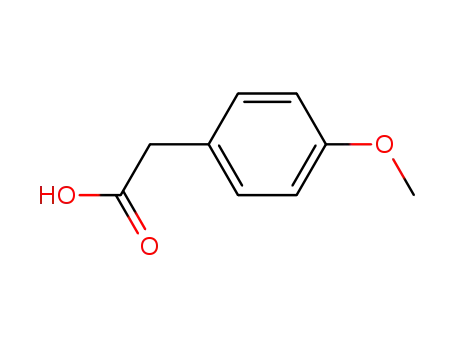
-
104-01-8
4-Methoxyphenylacetic acid

-
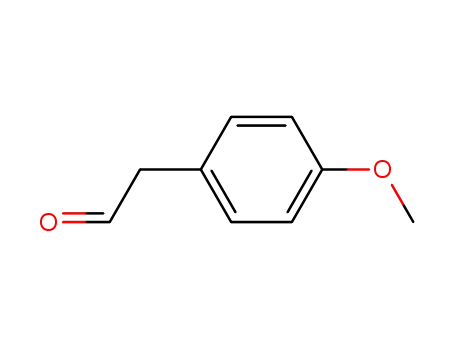
-
5703-26-4
4-Methoxyphenylacetaldehyde

-
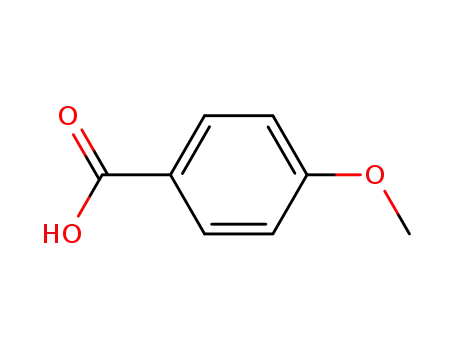
-
100-09-4
4-methoxybenzoic acid
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide; oxygen;
palladium on activated charcoal;
In
water;
at 90 ℃;
for 3h;
under 2280 Torr;
Product distribution;
Mechanism;
other alcohols; var. conditions, var. catalysts, additives;
|
36.2 % Chromat. 0.7 % Chromat. 24.6 % Chromat. |
-
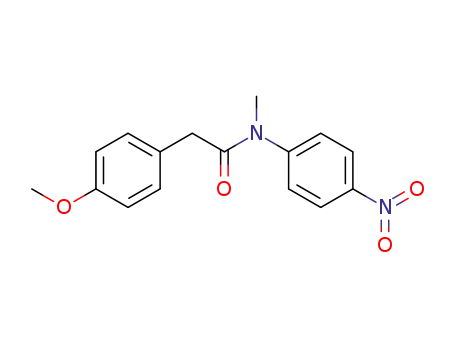
-
2-(4-Methoxy-phenyl)-N-methyl-N-(4-nitro-phenyl)-acetamide

-

-
104-01-8
4-Methoxyphenylacetic acid

-
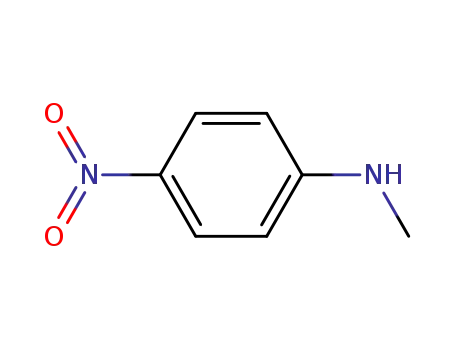
-
100-15-2
N-methyl(p-nitroaniline)
| Conditions | Yield |
|---|---|
|
With
barium dihydroxide; cetyltrimethylammonim bromide;
In
water;
at 30 ℃;
Rate constant;
Mechanism;
dependence of rate constant on concentration of cetyltrimethylammonium bromide;
|
104-01-8 Upstream products
-
140-67-0
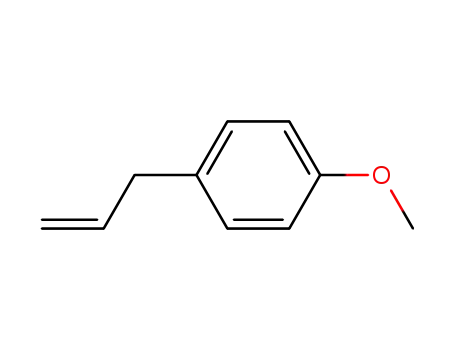
Estragole
-
33646-40-1
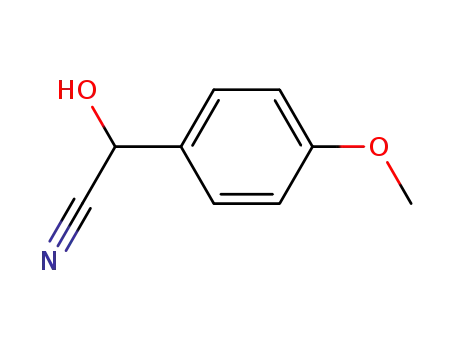
4-methoxymandelonitrile
-
5703-26-4

4-Methoxyphenylacetaldehyde
-
122-84-9
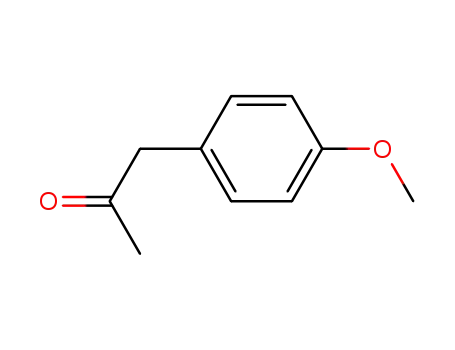
4-methoxybenzyl methyl ketone
104-01-8 Downstream products
-
4705-34-4
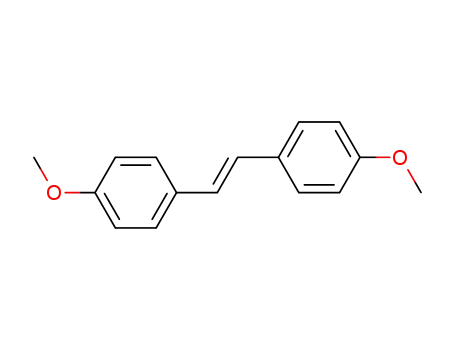
1,2-bis(4-methoxyphenyl)ethene
-
83307-72-6
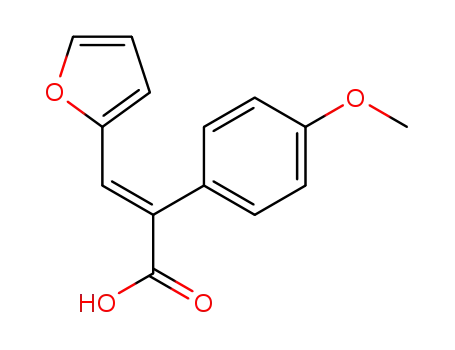
E-methyl-3-(2-furyl)-(2-p-methoxyphenyl) acrylic acid
-
68742-13-2
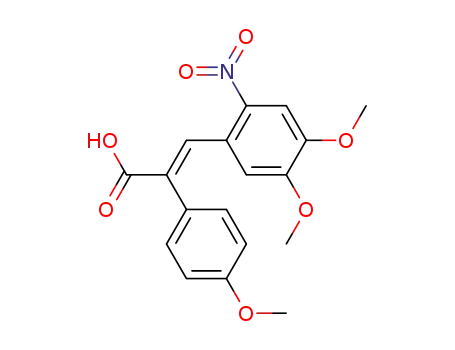
(2E)-3-(4,5-dimethoxy-2-nitrophenyl)-2-(4-methoxyphenyl)prop-2-enoic acid
-
68742-14-3
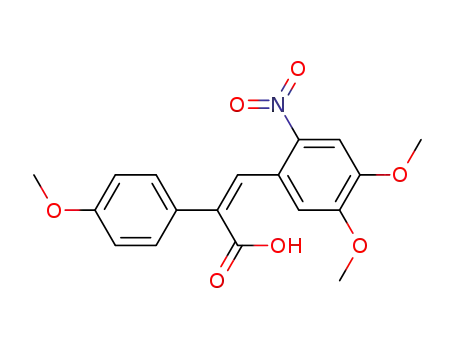
(2Z)-3-(4,5-dimethoxy-2-nitrophenyl)-2-(4-methoxyphenyl)prop-2-enoic acid
Relevant Products
-
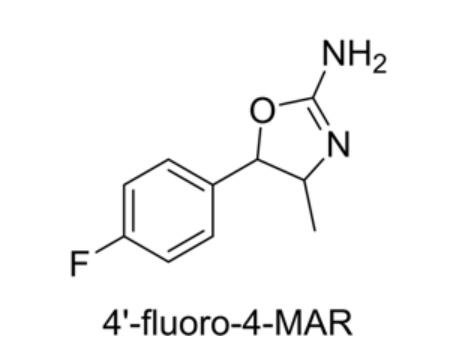
para-fluoro Methylaminorex
CAS:1364933-64-1
-
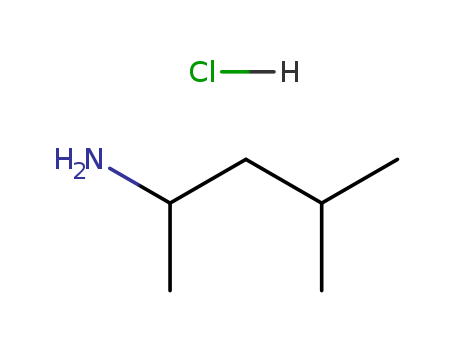
1,3-Dimethylbutylamine HCL
CAS:71776-70-0
-
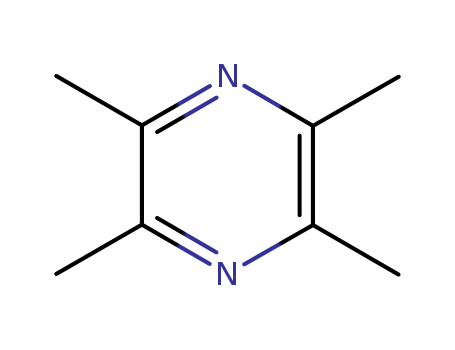
Tetramethylpyrazine
CAS:1124-11-4