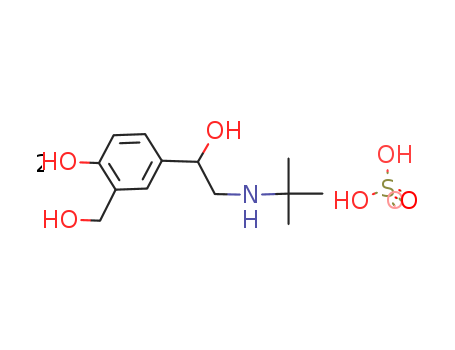
51022-70-9
- Product Name:Albuterol sulfate
- Molecular Formula:2(C13H21NO3).H2O4S
- Purity:99%
- Molecular Weight:576.709
Product Details;
CasNo: 51022-70-9
Molecular Formula: 2(C13H21NO3).H2O4S
Appearance: white crystalline solid
Albuterol sulfate Good Supplier In Bulk Supply High Purity 51022-70-9
- Molecular Formula:2C13H21NO3*H2O4S
- Molecular Weight:576.709
- Appearance/Colour:white crystalline solid
- Vapor Pressure:8.92E-08mmHg at 25°C
- Melting Point:180 °C
- Boiling Point:433.5 °C at 760 mmHg
- Flash Point:159.5 °C
- PSA:155.70000
- LogP:2.12490
Albuterol sulfate(Cas 51022-70-9) Usage
|
Biological Activity |
Non-selective β -adrenergic agonist, more potent at β 2 than β 1 receptors. |
|
Veterinary Drugs and Treatments |
Albuterol is used principally in dogs and cats for its effects on bronchial smooth muscle to alleviate bronchospasm or cough. It is also used in horses as a bronchodilator. |
|
Mode of action |
Albuterol Sulfate is the sulfate salt of the short-acting sympathomimetic agent albuterol, a 1:1 racemic mixture of (R)-albuterol and (S)-albuterol with bronchodilator activity. Albuterol stimulates beta2-adrenergic receptors in the lungs, thereby activating the enzyme adenylate cyclase that catalyzes the conversion of ATP to cyclic-3',5'-adenosine monophosphate (cAMP). Increased cAMP concentrations relax bronchial smooth muscle, relieve bronchospasms, and reduce inflammatory cell mediator release, especially from mast cells. To a lesser extent albuterol stimulates beta1-adrenergic receptors, thereby increasing the force and rate of myocardial contraction. |
|
Definition |
ChEBI: Albuterol sulfate is an ethanolamine sulfate salt. It is functionally related to an albuterol. |
|
Brand name |
Accuneb (Dey); Proair (IVAX); Proventil (Schering); Ventolin (GlaxoSmithKline); Volmax (Muro); Vospire (Odyssey). |
|
General Description |
Albuterol belongs to the class of medicines known as bronchodilators. It is also called a short-acting beta-agonist (SABA). |
InChI:InChI=1/2C12H19NO3.H2O4S/c2*1-12(2,3)13-7-11(16)8-4-5-9(14)10(15)6-8;1-5(2,3)4/h2*4-6,11,13-16H,7H2,1-3H3;(H2,1,2,3,4)
51022-70-9 Relevant articles
Process for the enantiomeric enrichment of salbutamol and salbutamol precursors
-
Page/Page column 10, (2008/06/13)
The present invention relates to a proce...
Drug delivery system
-
, (2008/06/13)
A drug delivery system in which a taste ...
51022-70-9 Process route
-
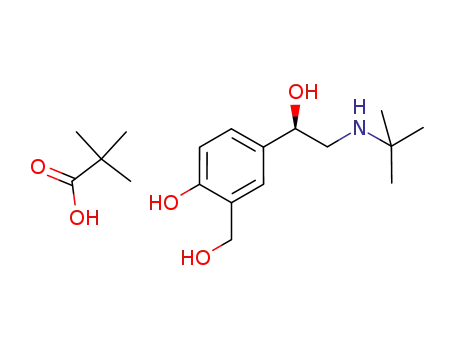
-
R-salbutamol*pivalic acid

-
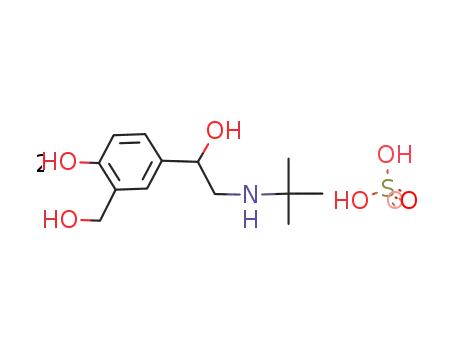
-
34245-12-0,36519-31-0,39971-61-4,51022-70-9,65143-06-8,148563-15-9,148563-16-0,324000-05-7,324000-04-6
salbutamol sulfate
| Conditions | Yield |
|---|---|
|
With
sulfuric acid;
In
methanol;
for 0.5h;
|
90% |

-

-
34245-12-0,36519-31-0,39971-61-4,51022-70-9,65143-06-8,148563-15-9,148563-16-0,324000-05-7,324000-04-6
salbutamol sulfate
| Conditions | Yield |
|---|---|
|
|
51022-70-9 Downstream products
-
324000-05-7
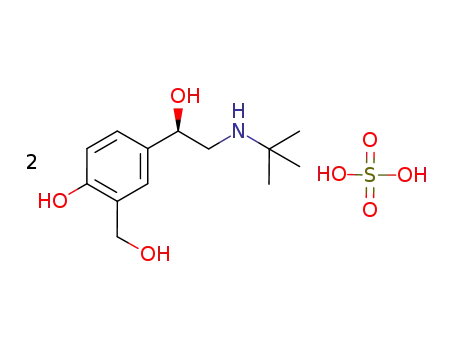
(R)-2-tert-butylamino-1-(4-hydroxy-2-hydroxymethyl-phenyl)ethanol
-
324000-05-7
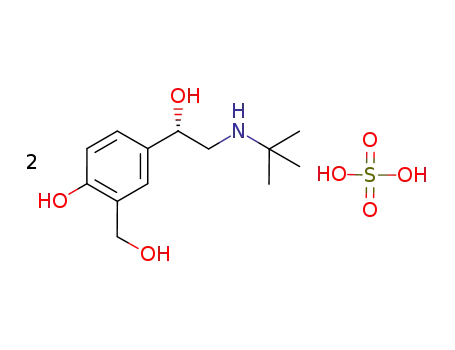
(S)-2-tert-butylamino-1-(4-hydroxy-2-hydroxymethyl-phenyl)ethanol
-
4199-09-1
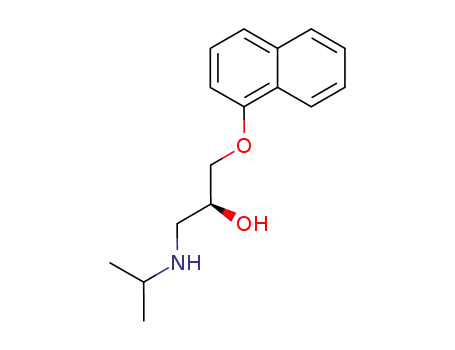
(S)-1-isopropylamino-3-(1-naphthyloxy)-2-propanol
-
525-66-6
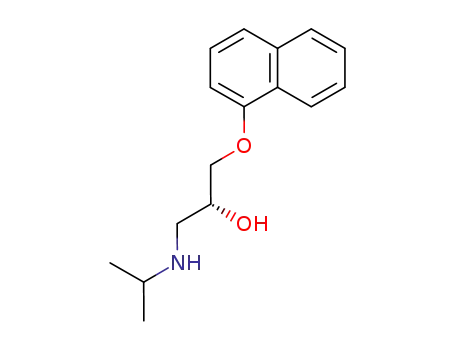
(R)-propranolol
Relevant Products
-
Chloramine-T trihydrate
CAS:7080-50-4
-
Cefixime
CAS:79350-37-1
-
4-Nitro-3-trifluoromethyl aniline
CAS:393-11-3