516-54-1
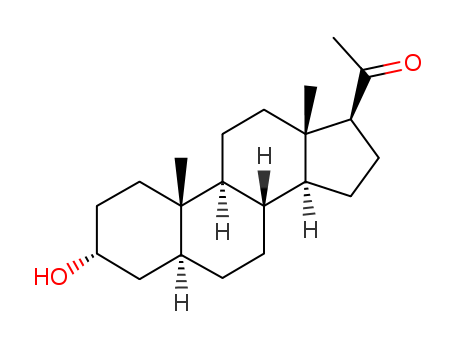
- Product Name:Brexanolone
- Molecular Formula:C21H34O2
- Purity:99%
- Molecular Weight:318.5
Product Details;
CasNo: 516-54-1
Molecular Formula: C21H34O2
Factory Supply Industrial Grade Brexanolone 516-54-1 with Best Price
- Molecular Formula:C21H34O2
- Molecular Weight:318.5
- Vapor Pressure:3.05E-09mmHg at 25°C
- Melting Point:176-178°
- Refractive Index:1.524
- Boiling Point:431.2°Cat760mmHg
- PKA:15.12±0.70(Predicted)
- Flash Point:183.9°C
- PSA:37.30000
- Density:1.053g/cm3
- LogP:4.59520
ALLOPREGNAN-3ALPHA-OL-20-ONE(Cas 516-54-1) Usage
InChI:InChI=1/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16-,17+,18-,19-,20-,21+/m0/s1
516-54-1 Relevant articles
Stereospecific reduction of 5β-reduced steroids by human ketosteroid reductases of the AKR (aldo-keto reductase) superfamily: Role of AKR1C1-AKR1C4 in the metabolism of testosterone and progesterone via the 5β-reductase pathway
Jin, Yi,Mesaros, A. Clementina,Blair, Ian A.,Penning, Trevor M.
experimental part, p. 53 - 61 (2012/06/15)
Active sex hormones such as testosterone...
METHODS OF NEUROPROTECTION USING NEUROPROTECTIVE STEROIDS AND A VITAMIN D
-
, (2012/01/03)
Described herein are compositions and me...
Reduction of steroidal ketones with amine - Boranes
Leontjev,Vasiljeva,Pivnitsky
, p. 703 - 708 (2007/10/03)
Complexes of secondary amines with boran...
REGIOSELECTIVE REDUCTION OF POLYKETONES ON SILICA GEL SURFACE WITH BORANE-TRIMETHYLAMINE COMPLEX
Gohzu, Shun-ichi,Tada, Masahiro
, p. 61 - 64 (2007/10/02)
Steroidal diones and trione, bicyclic di...
516-54-1 Process route
-
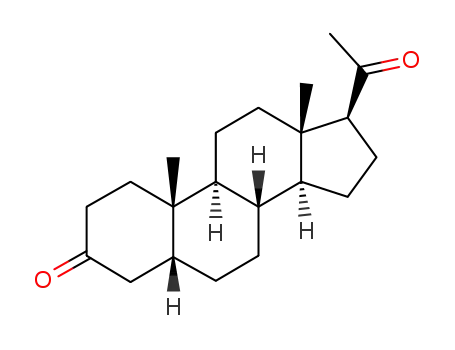
-
128-23-4
pregnanedione

-
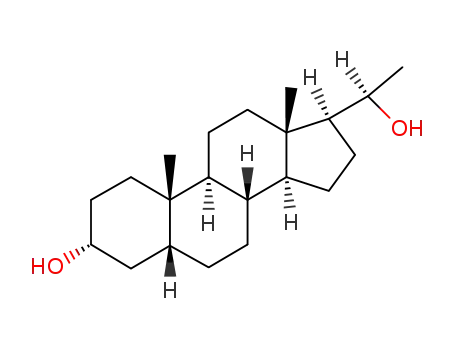
-
80-89-7,80-90-0,80-92-2,516-53-0,566-56-3,566-57-4,566-58-5,4406-36-4,4479-11-2,4707-80-6,6251-84-9,25908-35-4,28818-70-4,36027-66-4,38270-91-6,38270-97-2,52746-43-7,55569-11-4,55660-10-1,73745-18-3,98048-13-6,80-91-1
pregnadiol

-
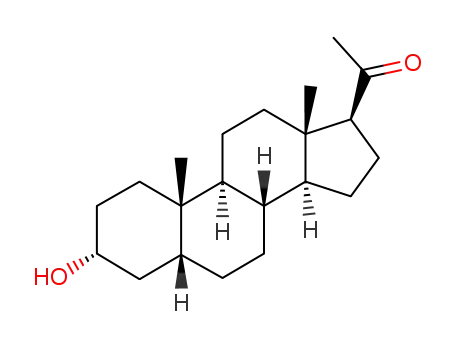
-
128-20-1,128-21-2,516-54-1,516-55-2,4243-94-1,4320-08-5,4406-35-3,4406-37-5,4469-03-8,13089-85-5,13089-86-6,14615-01-1,21788-58-9,24557-99-1,25126-79-8,26961-00-2,36027-63-1,39845-99-3,40135-22-6,72691-57-7,81076-02-0,95118-68-6,110350-90-8
PREGNANOLONE
| Conditions | Yield |
|---|---|
|
With
recombinant human aldo-keto reductase 1C3; NADPH;
In
methanol;
at 37 ℃;
for 1.5h;
pH=7;
stereospecific reaction;
aq. phosphate buffer;
Enzymatic reaction;
|
-
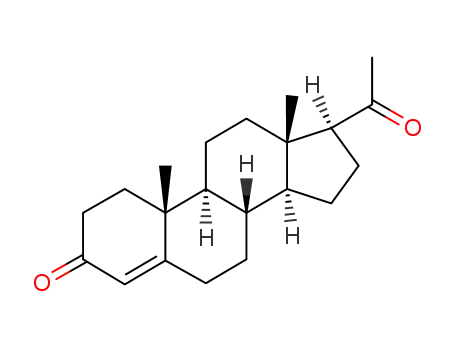
-
57-83-0
Progesterone

-

-
128-23-4
pregnanedione

-
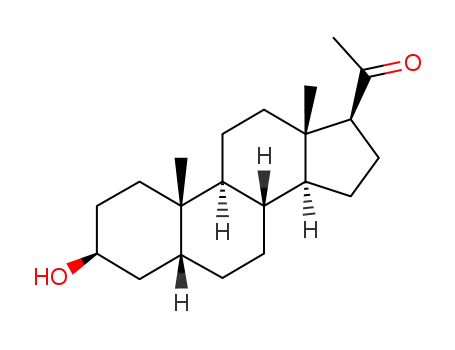
-
128-21-2
3β-hydroxy-5β-pregnan-20-one

-
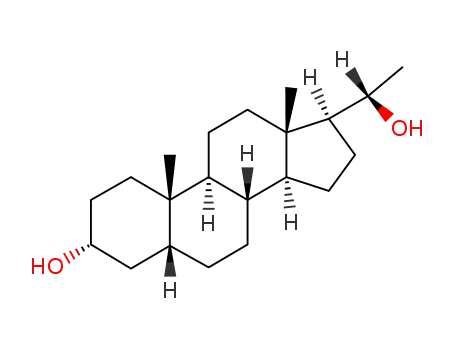
-
80-92-2
pregnanediol

-

-
80-89-7,80-90-0,80-92-2,516-53-0,566-56-3,566-57-4,566-58-5,4406-36-4,4479-11-2,4707-80-6,6251-84-9,25908-35-4,28818-70-4,36027-66-4,38270-91-6,38270-97-2,52746-43-7,55569-11-4,55660-10-1,73745-18-3,98048-13-6,80-91-1
pregnadiol

-

-
128-20-1,128-21-2,516-54-1,516-55-2,4243-94-1,4320-08-5,4406-35-3,4406-37-5,4469-03-8,13089-85-5,13089-86-6,14615-01-1,21788-58-9,24557-99-1,25126-79-8,26961-00-2,36027-63-1,39845-99-3,40135-22-6,72691-57-7,81076-02-0,95118-68-6,110350-90-8
PREGNANOLONE

-
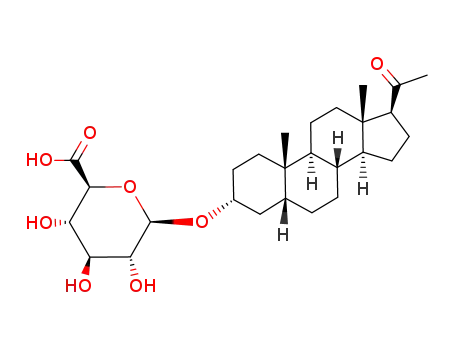
-
31329-54-1
3α-hydroxy-5β-pregnane-20-one-3-glucuronide
| Conditions | Yield |
|---|---|
|
With
phosphate buffer; (S)-2-[3]pyridyl-pyrrolidine-1-carboxylic acid amide; α-D-glucose 6-phosphate; bovine liver tissue supernatant; NADP; UDP-glucuronic acid;
at 37 ℃;
for 5h;
Product distribution;
<14C>labeled;
|
4 % Chromat. 1 % Chromat. 22 % Chromat. 15 % Chromat. 46 % Chromat. |
516-54-1 Upstream products
-
566-65-4
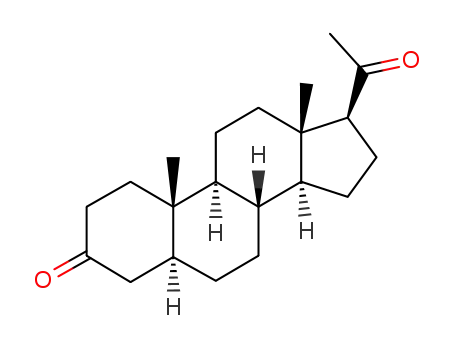
dihydroprogesterone
-
57-83-0

Progesterone
-
10035-10-6
hydrogen bromide
-
64-19-7
acetic acid
516-54-1 Downstream products
-
906-83-2
Pregnanolon-3β-acetat
-
1852-53-5
androstanediol
-
862259-66-3
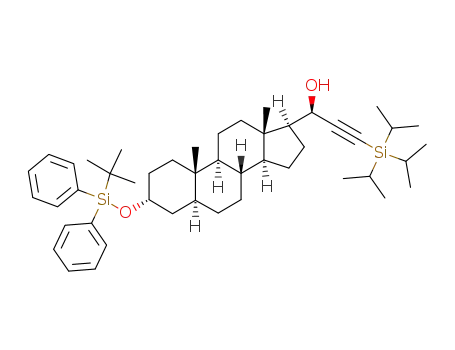
(20R)-1-[3α-(tert-butyldiphenylsilyloxy)-5α-androstan-17β-yl]-3-triisopropylsilyl-2-propyn-1-ol
-
862259-74-3
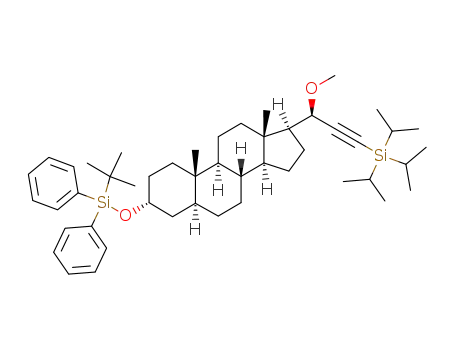
(20R)-3-triisopropylsilyl-1-methoxy-1-[3α-(tert-butyldiphenylsilyloxy)-5α-androstan-17β-yl]-2-propyne
Relevant Products
-
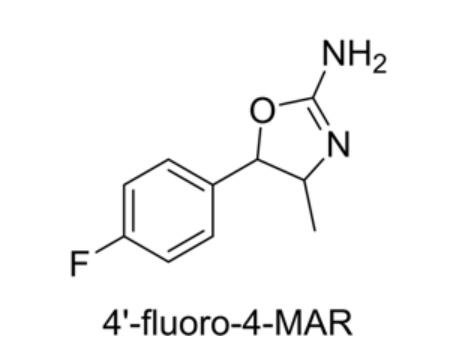
para-fluoro Methylaminorex
CAS:1364933-64-1
-
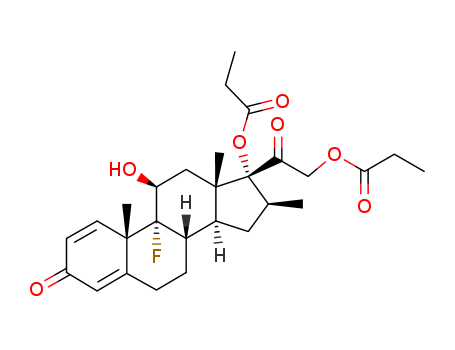
Betamethasone Dipropionate
CAS:5593-20-4
-
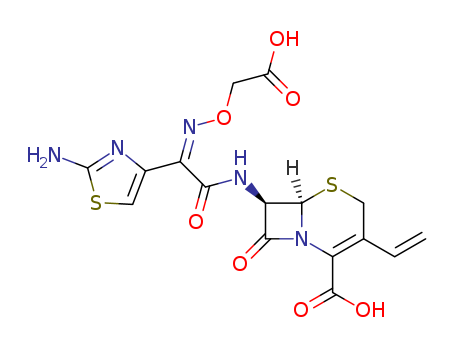
Cefixime
CAS:79350-37-1