673-31-4
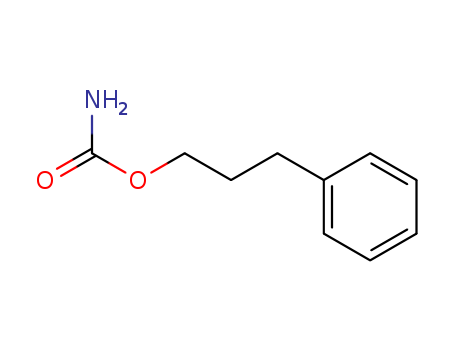
- Product Name:Phenprobamate
- Molecular Formula:C10H13 N O2
- Purity:99%
- Molecular Weight:179.219
Product Details;
CasNo: 673-31-4
Molecular Formula: C10H13 N O2
Top Quality Phenprobamate 673-31-4 Hot Sell In Stock
- Molecular Formula:C10H13 N O2
- Molecular Weight:179.219
- Vapor Pressure:4.69E-05mmHg at 25°C
- Melting Point:101-104°
- Refractive Index:1.5710 (estimate)
- Boiling Point:349.5oC at 760 mmHg
- PKA:13.47±0.50(Predicted)
- Flash Point:183.1oC
- PSA:52.32000
- Density:1.103g/cm3
- LogP:2.41480
Phenprobamate(Cas 673-31-4) Usage
|
Safety Profile |
Poison by intravenous and intraperitoneal routes. Moderately toxic by ingestion. Used as a tranquilizer and muscle relaxant. When heated to decomposition it emits toxic fumes of NOx. See also CARBAMATES and ESTERS |
|
Definition |
ChEBI: Phenprobamate is a member of benzenes. |
InChI:InChI=1/C10H13NO2/c11-10(12)13-8-4-7-9-5-2-1-3-6-9/h1-3,5-6H,4,7-8H2,(H2,11,12)
673-31-4 Relevant articles
Further development of the tin-catalyzed transcarbamoylation reaction
Hasegawa, Tomoyuki,Ichikawa, Yoshiyasu,Masuda, Toshiya,Minami, Takahiro,Morishita, Yukinori,Ochi, Rika,Sato, Hiroshi
supporting information, p. 2373 - 2378 (2020/08/19)
Studies carried out to further develop t...
Tuning Triplet Energy Transfer of Hydroxamates as the Nitrene Precursor for Intramolecular C(sp3)-H Amidation
Chang, Sukbok,Jung, Hoimin,Keum, Hyeyun,Kweon, Jeonguk
, p. 5811 - 5818 (2020/04/10)
Reported herein is the design of a photo...
Copper-catalyzed intramolecular C-H amination
Barman, Dipti N.,Nicholas, Kenneth M.
, p. 908 - 911 (2011/04/26)
The amino-functionalization of tertiary,...
An efficient synthesis of carbamates by tin-catalyzed transcarbamoylation reactions of primary and secondary alcohols
Ichikawa, Yoshiyasu,Morishita, Yukinori,Kusaba, Shuhei,Sakiyama, Naoto,Matsuda, Yasunori,Nakano, Keiji,Kotsuki, Hiyoshizo
experimental part, p. 1815 - 1818 (2010/10/05)
A new approach to the synthesis of carba...
673-31-4 Process route
-
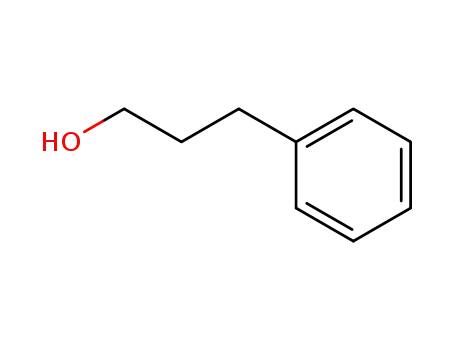
-
122-97-4
3-Phenyl-1-propanol

-
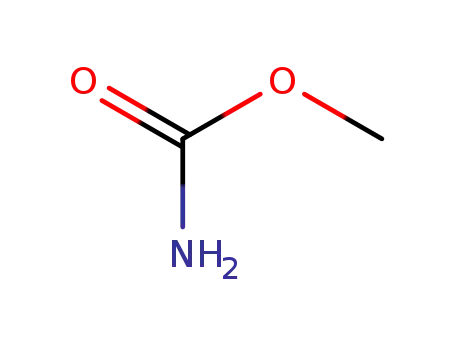
-
598-55-0
methyl carbamate

-
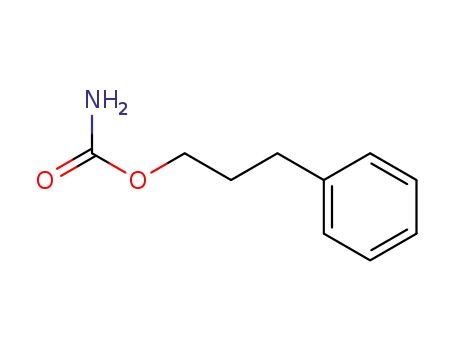
-
673-31-4
phenprobamate
| Conditions | Yield |
|---|---|
|
With
di-n-butyltin maleate;
In
toluene;
for 3h;
Reflux;
|
98% |
-

-
122-97-4
3-Phenyl-1-propanol

-
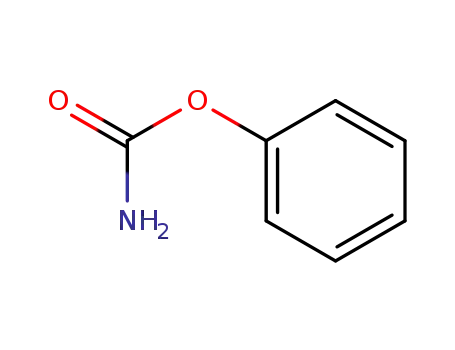
-
622-46-8
carbamic acid phenyl ester

-

-
673-31-4
phenprobamate
| Conditions | Yield |
|---|---|
|
With
di-n-butyltin maleate;
In
toluene;
at 90 ℃;
for 4h;
|
99% |
673-31-4 Upstream products
-
122-97-4

3-Phenyl-1-propanol
-
143-33-9
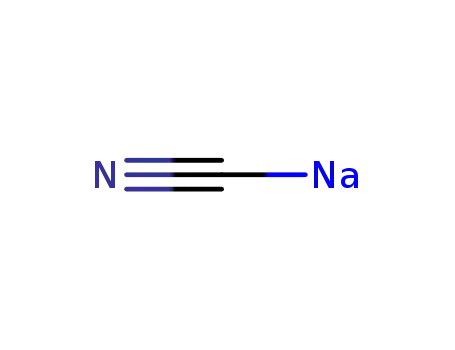
sodium cyanide
-
622-46-8

carbamic acid phenyl ester
-
598-55-0

methyl carbamate
673-31-4 Downstream products
-
41142-04-5
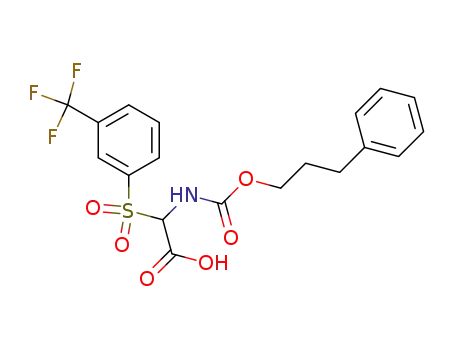
(3-Phenyl-propoxycarbonylamino)-(3-trifluoromethyl-benzenesulfonyl)-acetic acid
-
41142-18-1
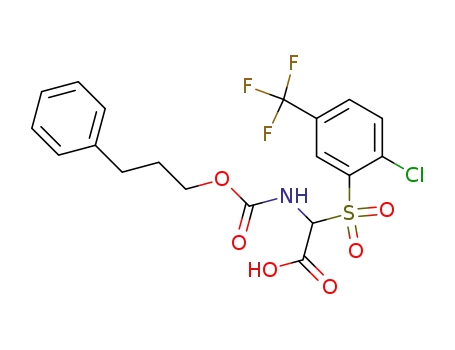
(2-Chloro-5-trifluoromethyl-benzenesulfonyl)-(3-phenyl-propoxycarbonylamino)-acetic acid
-
1218988-28-3
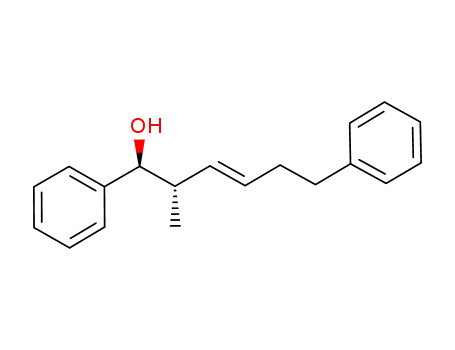
(1S,2S,3E)-2-methyl-1,6-diphenylhex-3-en-1-ol
-
1218988-13-6
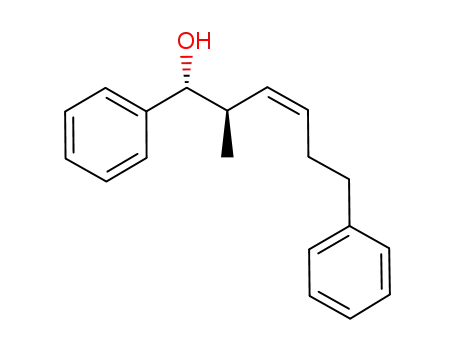
(1R,2R,3Z)-2-methyl-1,6-diphenylhex-3-en-1-ol
Relevant Products
-
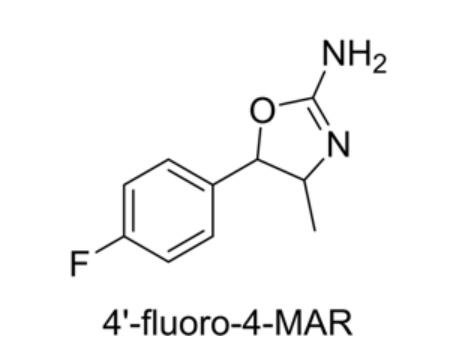
para-fluoro Methylaminorex
CAS:1364933-64-1
-
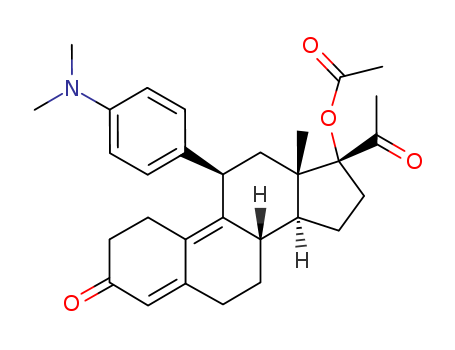
Ulipristal acetate
CAS:126784-99-4
-
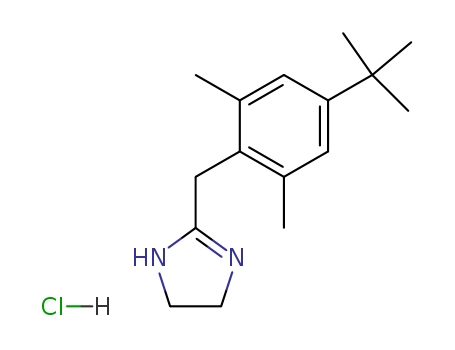
Xylometazoline hydrochloride
CAS:1218-35-5