4105-38-8
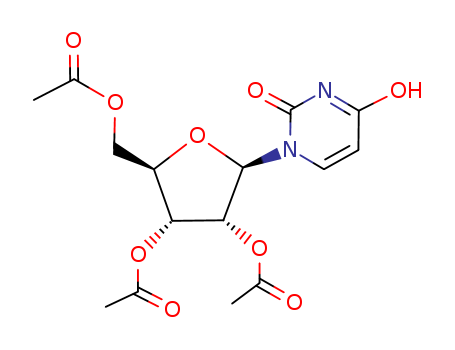
- Product Name:2',3',5'-Tri-O-acetyluridine; Uridine Triacetate
- Molecular Formula:C15H18N2O9
- Purity:99%
- Molecular Weight:370.316
Product Details;
CasNo: 4105-38-8
Molecular Formula: C15H18N2O9
Appearance: white or almost white crystalline powder
Quality Factory Sells Top Purity 99% 2',3',5'-Tri-O-acetyluridine; Uridine Triacetate 4105-38-8 with Safe Delivery
- Molecular Formula:C15H18N2O9
- Molecular Weight:370.316
- Appearance/Colour:white or almost white crystalline powder
- Melting Point:124-134oC
- Refractive Index:1.552
- PKA:9.39±0.10(Predicted)
- PSA:142.99000
- Density:1.43 g/cm3
- LogP:-1.13950
2',3',5'-Tri-O-acetyluridine(Cas 4105-38-8) Usage
|
Synthesis |
Commercially available uridine (167) was treated with acetic anhydride in the presence of catalytic boron trifluoride-etherate, and the crude product was recrystallized from ethanol to give uridine triacetate (XX) in 74-78% yield. |
|
Definition |
ChEBI: An acetate ester that is uracil in which the three hydroxy hydrogens are replaced by acetate group. A prodrug for uridine, it is used for the treatment of hereditary orotic aciduria and for management of fluorouracil toxicity. |
InChI:InChI=1/C15H18N2O9/c1-7(18)23-6-10-12(24-8(2)19)13(25-9(3)20)14(26-10)17-5-4-11(21)16-15(17)22/h4-5,10,12-14H,6H2,1-3H3,(H,16,21,22)
4105-38-8 Relevant articles
Anomalous interaction of tri-acyl ester derivatives of uridine nucleoside with a l-α-dimyristoylphosphatidylcholine biomembrane model: a differential scanning calorimetry study
Berrío Escobar, Jhon Fernando,Márquez Fernández, Diana Margarita,Giordani, Cristiano,Castelli, Francesco,Sarpietro, Maria Grazia
, p. 329 - 337 (2019)
Objectives: Uridine was conjugated with ...
NMR studies show monomeric 5-fluorouridine forms base pairs of increased stability compared with uridine in non-aqueous solvents
Gmeiner,Anderson,Sahasrabudhe
, p. 2329 - 2344 (1994)
The binding constants and geometries for...
Chemoenzymatic synthesis of Park's nucleotide: toward the development of high-throughput screening for MraY inhibitors
Kurosu, Michio,Mahapatra, Sebabrata,Narayanasamy, Prabagaran,Crick, Dean C.
, p. 799 - 803 (2007)
An efficient chemoenzymatic synthesis of...
Optimized synthesis of [3-15N]-labeled uridine phosphoramidites
Baral, Bharat,Kumar, Pawan,Anderson, Brooke A.,?stergaard, Michael E.,Sharma, Pawan K.,Hrdlicka, Patrick J.
, p. 5850 - 5852 (2009)
A short and high-yielding synthetic rout...
-
Rajabalee
, p. 75 (1971)
-
Cyclic sulfates as synthetic equivalents of α-epoxynucleosides
Serra, Carme,Farras, Jaume,Vilarrasa, Jaume
, p. 9111 - 9113 (1999)
A stable sulfate derivative of N-nitrour...
Anti-hepatitis B virus compound as well as preparation method and application thereof
-
Paragraph 0025-0028, (2021/06/22)
The invention provides an anti-hepatitis...
Transglycosylation in the Modification and Isotope Labeling of Pyrimidine Nucleosides
Gong, Yong,Chen, Lu,Zhang, Wei,Salter, Rhys
supporting information, p. 5577 - 5581 (2020/07/24)
Transglycosylation of pyrimidine nucleos...
Stereoselective Synthesis of Highly Functionalized Arabinosyl Nucleosides through Application of an N-Nitro Protecting Group
Hilko, David H.,Bornaghi, Laurent F.,Poulsen, Sally-Ann
supporting information, p. 11944 - 11955 (2018/09/25)
2′-Deoxy-2′,5-disubstituted arabinosyl u...
4105-38-8 Process route
-
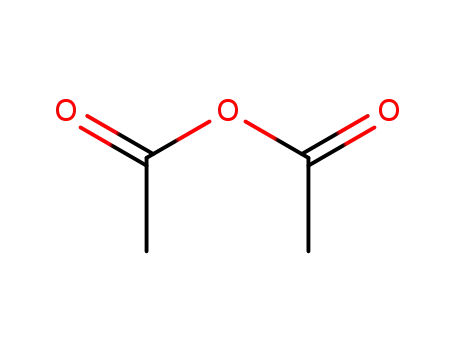
-
108-24-7
acetic anhydride

-
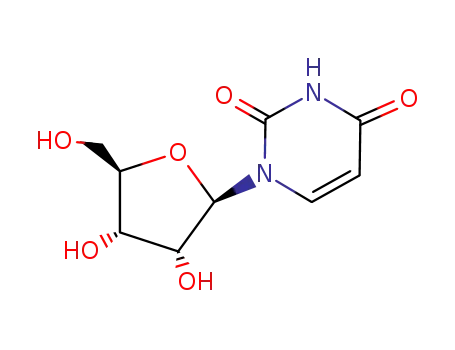
-
58-96-8
uridine

-
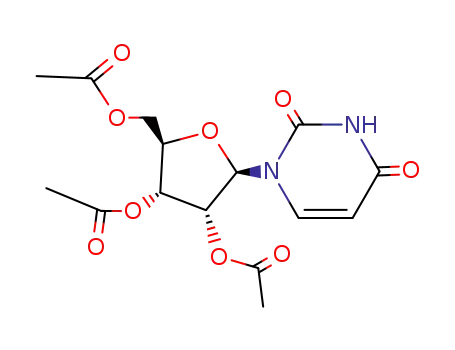
-
4105-38-8
Tri-O-acetyluridine
| Conditions | Yield |
|---|---|
|
With
dmap;
|
100% |
|
With
triethylamine;
In
1,4-dioxane;
at 25 ℃;
for 36h;
|
100% |
|
With
pyridine;
at 20 ℃;
for 16h;
|
100% |
|
With
triethylamine;
In
N,N-dimethyl-formamide;
at 20 ℃;
|
100% |
|
With
pyridine;
Cooling with ice;
|
99% |
|
With
pyridine;
at 20 ℃;
Cooling with ice;
|
98% |
|
With
dmap; triethylamine;
In
acetonitrile;
at 20 ℃;
|
96% |
|
With
pyridine; dmap;
at 20 ℃;
|
95% |
|
With
dmap;
In
pyridine;
at 4 - 20 ℃;
for 24h;
Inert atmosphere;
|
95% |
|
With
pyridine; dmap;
at 25 ℃;
for 4h;
|
94% |
|
With
lithium perchlorate;
for 10h;
Heating;
|
94% |
|
With
dmap;
In
acetonitrile;
for 1h;
Ambient temperature;
|
93% |
|
molecular sieve; potassium chloride;
at 100 ℃;
for 1.5h;
|
93% |
|
With
dmap; 1-ethylene glycol monomethyl ether-3-methylimidazolium methanesulfonate;
at 20 ℃;
for 0.416667h;
|
93% |
|
In
pyridine;
at 60 ℃;
for 3h;
|
92% |
|
acetic anhydride;
With
molybdenium(VI) dioxodichloride;
In
dichloromethane;
at 20 ℃;
for 0.5h;
uridine;
In
dichloromethane;
at 20 ℃;
for 107h;
|
92% |
|
In
neat (no solvent);
Molecular sieve;
Microwave irradiation;
Green chemistry;
|
92% |
|
With
vanadyl triflate;
In
dichloromethane;
at 20 ℃;
for 96h;
|
90% |
|
With
iron(III) sulfate;
at 20 ℃;
for 7h;
|
89% |
|
With
pyridine;
at 60 ℃;
for 3h;
|
88% |
|
In
pyridine;
at 20 ℃;
Inert atmosphere;
|
84% |
|
With
pyridine;
at 60 ℃;
for 3h;
|
79% |
|
With
pyridine;
at 60 ℃;
for 3h;
|
79% |
|
With
pyridine;
at 60 ℃;
for 3h;
|
79% |
|
With
sodium acetate;
|
|
|
In
pyridine;
|
|
|
With
pyridine;
at 20 ℃;
for 1h;
|
|
|
With
pyridine;
for 4h;
Ambient temperature;
|
|
|
With
pyridine;
|
|
|
With
pyridine;
at 60 ℃;
|
|
|
With
dmap; triethylamine;
In
acetonitrile;
at 20 ℃;
for 1h;
|
|
|
In
pyridine;
|
|
|
In
1,4-dioxane;
|
|
|
With
pyridine;
at 0 ℃;
for 5h;
|
|
|
With
pyridine;
for 5h;
|
|
|
|
-
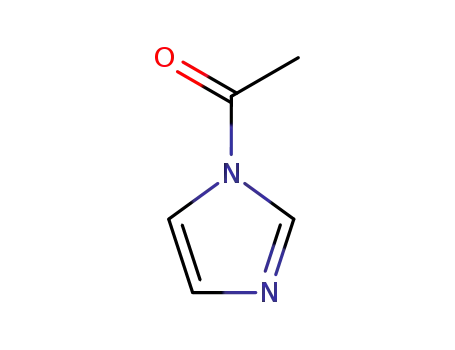
-
2466-76-4
N-Acetylimidazole

-

-
58-96-8
uridine

-

-
4105-38-8
Tri-O-acetyluridine
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 4h;
pH=8;
|
74% |
4105-38-8 Upstream products
-
108-24-7

acetic anhydride
-
58-96-8

uridine
-
144102-56-7
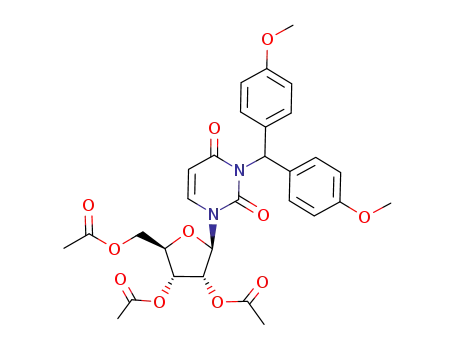
Acetic acid (2R,3R,4R,5R)-4-acetoxy-5-acetoxymethyl-2-{3-[bis-(4-methoxy-phenyl)-methyl]-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl}-tetrahydro-furan-3-yl ester
-
117901-67-4
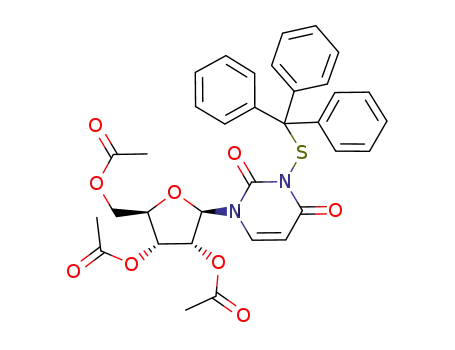
Acetic acid (2R,3R,4R,5R)-4-acetoxy-5-acetoxymethyl-2-(2,4-dioxo-3-tritylsulfanyl-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-3-yl ester
4105-38-8 Downstream products
-
60543-63-7
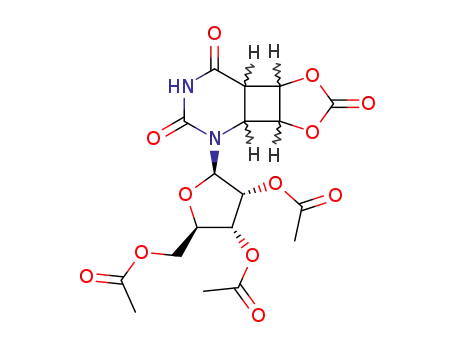
2',3',5'-Tri-O-acetyluridin-Vinylencarbonat-Addukt
-
98889-52-2
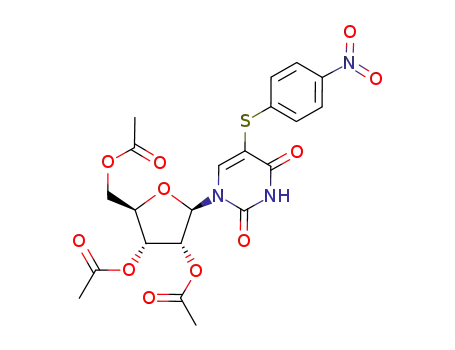
5-(4-nitrobenzene)sulfenyl-2',3',5'-tri-O-acetyluridine
-
98889-50-0
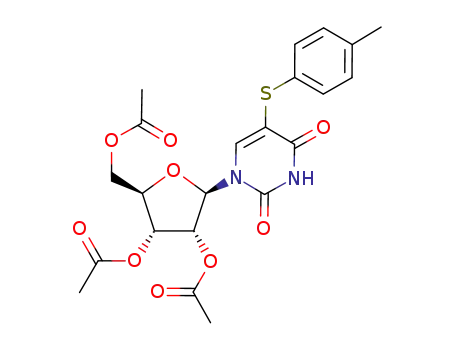
5-(4-toluene)sulfenyl-2',3',5'-tri-O-acetyluridine
-
93960-48-6
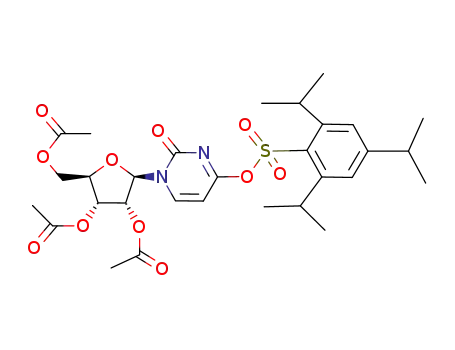
2',3',5'-tri-O-acetyl-4-O-(2,4,6-triisopropylbenzenesulfonyl)-uridine
Relevant Products
-
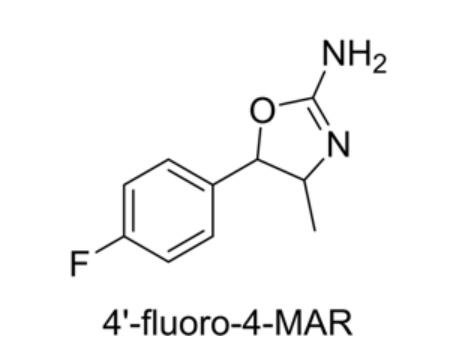
para-fluoro Methylaminorex
CAS:1364933-64-1
-
Linezolid
CAS:165800-03-3
-
Ulipristal acetate
CAS:126784-99-4