657-24-9
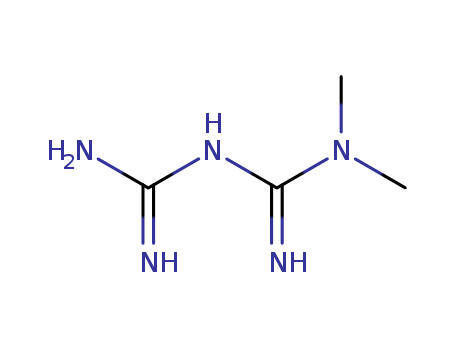
- Product Name:Metformin
- Molecular Formula:C4H11N5
- Purity:99%
- Molecular Weight:129.165
Product Details;
CasNo: 657-24-9
Molecular Formula: C4H11N5
Purity 99% Min Metformin 657-24-9 Spot Supply with Safe Transportation
- Molecular Formula:C4H11N5
- Molecular Weight:129.165
- Melting Point:199-200 °C
- Refractive Index:1.5760 (estimate)
- Boiling Point:224.1 °C at 760 mmHg
- PKA:pKa 2.8(H2O,t =32) (Uncertain)
- Flash Point:89.3 °C
- PSA:88.99000
- Density:1.28 g/cm3
- LogP:0.25650
Metformin(Cas 657-24-9) Usage
|
History |
Metformin is a biguanide compound which originated from the extraction of goat’s rue plants. The structure of metformin was identified by British scholars in the early 1920s. In 1922, Werner and Bell et?al. first synthesized metformin in 31 institutes in Dublin, Ireland. In 1929, Slotta and Tschesche found metformin’s hypoglycemic action. However, because of other potent antidiabetic drugs such as insulin which were widely used in clinical practice, the pharmacological effects of metformin didn’t receive much attention.Until the 1950s, a French diabetic scientist Jean Sterne found the hypoglycemic effect of metformin through the study of galegine. Then the drug was used in diabetic patients for the first time, and the results were published in 1957. UKPDS, which began from 1977 and ended in 1997 and was then followed up for 10?years, is the longest in the history of clinical trials and has a significant impact on practice and guidelines for prevention and treatment of diabetes mellitus. In this trial, metformin was found to reduce the risk of diabetic complications by 32%. In addition, it was proved for the first time that metformin can reduce blood glucose and protect against cardiovascular function, especially in obese patients. In 1994, metformin was approved by the US FDA for type 2 diabetes treatment. Currently, metformin has become the world’s most widely used antidiabetic drug.Aiming at improving the stability of the absorption of metformin, chemists have also carried out a series of structural renovation and modification. Metformin activates with carbonyl, esters, chlorides, and aldehydes to form triazine compounds, with 1,3-diketone to produce pyrimidine compounds, and with disulfides to produce C-S coupling products, etc. |
|
Indications |
Metformin (Glucophage) was used in Europe for many years before it was approved for use in the United States in 1995. Metformin is the only approved biguanide for the treatment of patients with NIDDM that are refractory to dietary management alone. Metformin does not affect insulin secretion but requires the presence of insulin to be effective. The exact mechanism of metformin’s action is not clear, but it does decrease hepatic glucose production and increase peripheral glucose uptake. When used as monotherapy, metformin rarely causes hypoglycemia. |
|
Therapeutic Function |
Oral hypoglycemic |
|
Biological Functions |
Metformin can lower free fatty acid concentrations by 10 to 30%. This antilipolytic effect may help to explain the reduction in gluconeogenesis through reduced levels of available substrate (65). When given as a monotherapy, metformin treatment does not lead to hypoglycemia, so it is better described as an antihyperglycemic agent rather than a hypoglycemic agent. |
|
Pharmacology |
As a traditional antidiabetic drug, metformin can reduce the levels of blood glucose and lipid, as well as regulating cell growth, anti-inflammation, antiaging, etc. The main pharmacological mechanisms are inhibition of hepatic gluconeogenesis, the activation of AMP-activated protein kinase (AMPK), and the regulation of mitochondrial function. Improvement of Insulin Resistance and Decrease of Blood Glucose Levels The main pharmacological effects of biguanide drugs are to reduce blood glucose output and improve peripheral insulin resistance. Metformin inhibits hyperglycemia mainly by inhibiting hepatic glucose production (hepatic gluconeogenesis), increasing muscle glucose uptake. Regulation of Lipid Metabolism and Reducing Body Weight Metformin can reduce the blood triglycerides in circulation, improve liver steatosis, promote the oxidation of brown adipose tissue and VLDL-fatty acid triglyceride uptake, and inhibit fat formation; the process may be related with the activation of AMPK. AMPK can phosphorylate acetyl coenzyme A carboxylase (ACC) and inhibit the conversion of acetyl coenzyme A into malonyl-CoA. Malonyl-CoA is a precursor of fatty acid production and is an allosteric inhibitor of fatty acid transporting to mitochondria. Prevention and Treatment of Tumors Epidemiological studies have shown that metformin reduced the risk of multiple types of tumors in type 2 diabetes and nondiabetes patients and reduced tumor-related mortality. Metformin also has a therapeutic effect on various tumors. The antitumor effect of metformin may be through the reduction of serum insulin and insulin-like growth factor-1 (IGF-1) levels or activation of LKB1/AMPK, thereby blocking the mammalian target of rapamycin-sensitive complex 1 (mTORC1) signaling pathway. Antiaging Antiaging effect is a major discovery in the new role of metformin. FDA approved the phase 4 of clinical trial of metformin for antiaging effect, and the trial is ongoing. The study suggests that the antiaging effect of metformin is closely related to the mitochondria, through the regulation of mitochondrial function to activate AMPK and inhibit mTOR, thereby reducing energy consumption. Furthermore, metformin can regulate oxidative stress, reduce tissue inflammation, and reduce the growth factor level and cell proliferation. The above effects and the combinational effect result in the improvement of health and achieving longevity. Other Effects Studies have shown that metformin helps to prevent type 2 diabetes. For young people with a high body mass index, metformin is more effective than lifestyle control. In addition, metformin can improve and prevent vascular disease, improve mitochondrial function, and serve as anti-inflammatory agent and antioxidant. Metformin is hydrophilic and distributed in tissues in an active manner. After oral administration, metformin is absorbed quickly and rapidly distributed into various tissues, mainly in the liver, gastrointestinal tract, and kidney. |
|
Safety Profile |
Poison by subcutaneous and intraperitoneal routes. Mildly toxic by parenteral route. Experimental teratogenic effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx |
|
Metabolism |
Metformin is quickly absorbed from the small intestine. Bioavailability is from 50 to 60%, and the drug is not protein bound. Peak plasma concentrations occur at approximately 2 hours. The drug is widely distributed in the body and accumulates in the wall of the small intestine. This depot of drug serves to maintain plasma concentrations. Metformin is excreted in the urine, via tubular excretion, as unmetabolized drug with a half-life of approximately 2 to 5 hours; therefore, renal impairment as well as hepatic disease are contraindications for the drug. |
|
Physical properties |
Appearance: white crystalline or crystalline powder, odorless. Solubility: freely soluble in water, soluble in methanol, slightly soluble in ethanol, and insoluble in chloroform or ether. Melting point: 223–226°C. |
|
Definition |
ChEBI: Metformin is a member of the class of guanidines that is biguanide the carrying two methyl substituents at position 1. It has a role as a hypoglycemic agent, a xenobiotic, an environmental contaminant and a geroprotector. It is functionally related to a biguanide. It is a conjugate base of a metformin(1+). |
|
General Description |
Metformin, N,N-dimethylimidodicarbonimidicdiamide hydrochloride (Glucophage), is a bisguanidine.This class of agents is capable of reducing sugar absorptionfrom the gastrointestinal tract. Also, they can decrease gluconeogenesiswhile increasing glucose uptake by muscles andfat cells. These effects, in turn, lead to lower blood glucoselevels. Unlike the sulfonylureas, these are not hypoglycemicagents but rather can act as antihyperglycemics. This differencein nomenclature is caused by the inability of these agentsto stimulate the release of insulin from the pancreas. Often,metformin is coadministered with the nonsulfonylureas to improvethe efficacy of those agents. |
InChI:InChI=1/C4H11N5/c1-9(2)4(7)8-3(5)6/h1-2H3,(H5,5,6,7,8)
657-24-9 Relevant articles
A ternary tetracoordinated PdII complex with metformin and dipicolinate: Synthesis, characterization and crystal structure
Moghimi,Khavassi,Dashtestani,Kordestani,Ekram Jafari,Maddah,Moosavi
, p. 38 - 41 (2011)
A proton transfer compound L, (MetH)2(di...
Novel halogenated sulfonamide biguanides with anti-coagulation properties
Huttunen, Kristiina M.,Markowicz-Piasecka, Magdalena,Sikora, Joanna,Zajda, Agnieszka
, (2019)
Apart from its hypoglycaemic properties,...
Convenient microwave-assisted synthesis of lipophilic sulfenamide prodrugs of metformin
Huttunen, Kristiina M.,Lepp?nen, Jukka,Laine, Krista,Veps?l?inen, Jouko,Rautio, Jarkko
, p. 624 - 628 (2013)
A convenient microwave-assisted synthesi...
Solid state and solubility study of a potential anticancer drug-drug molecular salt of diclofenac and metformin
Feng, Wen-Quan,Wang, Ling-Yang,Gao, Jie,Zhao, Ming-Yu,Li, Yan-Tuan,Wu, Zhi-Yong,Yan, Cui-Wei
, (2021)
To improve the physicochemical propertie...
Two N,N-dimethylbiguanidium salts displaying double hydrogen bonds to the counter-ions
Lu, Li-Ping,Zhang, Hong-Mei,Feng, Si-Si,Zhu, Miao-Li
, (2004)
An investigation into the crystal struct...
Novel sulfonamide-based analogs of metformin exert promising anti-coagulant effects without compromising glucose-lowering activity
Markowicz-Piasecka, Magdalena,Sadkowska, Adrianna,Sikora, Joanna,Broncel, Marlena,Huttunen, Kristiina M.
, p. 1 - 28 (2020)
Metformin, one of the most frequently pr...
Sulfonamide metformin derivatives induce mitochondrial-associated apoptosis and cell cycle arrest in breast cancer cells
Huttunen, Johanna,Huttunen, Kristiina M.,Markowicz-Piasecka, Magdalena,Sikora, Joanna,Zajda, Agnieszka
, (2021/12/31)
Metformin, an oral anti-diabetic drug, h...
5-substituted 2,4-thiazolidinediones (thiohydantoins), pseudothiohydantoins, and propseudothiohydantoins for use as antiviral agents
-
Page/Page column 13-14, (2020/06/03)
The present invention concerns the synth...
657-24-9 Process route
-
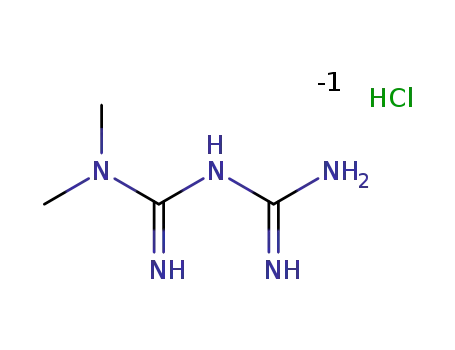
-
N,N-dimethylimidodicarbonimidic diamide hydrochloride

-
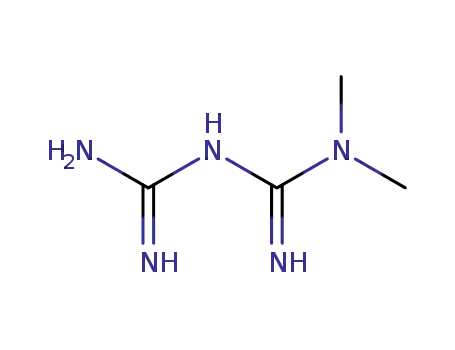
-
657-24-9
dimethylbiguanide
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
isopropyl alcohol;
at 0 - 40 ℃;
for 18.2h;
Temperature;
Solvent;
|
93% |
|
With
sodium hydroxide;
In
isopropyl alcohol;
at 39.84 ℃;
for 3h;
|
|
|
With
sodium;
In
ethanol;
at 60 ℃;
for 1h;
Reagent/catalyst;
Solvent;
Temperature;
|
-
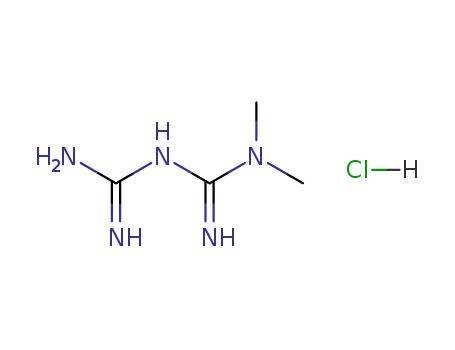
-
1115-70-4
metformin hydrochloride

-

-
657-24-9
dimethylbiguanide
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 0.5h;
|
100% |
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 0.5h;
|
99% |
|
With
sodium hydroxide;
In
ethanol;
for 5h;
|
99% |
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 0.5h;
Reagent/catalyst;
Temperature;
Solvent;
|
99% |
|
With
sodium hydroxide;
In
ethanol;
at 20 ℃;
for 1h;
|
99% |
|
With
potassium hydroxide;
In
isopropyl alcohol;
at 50 ℃;
for 2h;
|
98.5% |
|
With
potassium hydroxide;
In
isopropyl alcohol;
at 50 ℃;
for 2h;
|
98% |
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 0.5h;
|
95% |
|
With
sodium hydroxide;
In
ethanol;
at 70 ℃;
Product distribution / selectivity;
|
83.4% |
|
With
sodium hydroxide;
In
ethanol; dichloromethane;
at 70 ℃;
Product distribution / selectivity;
|
83.4% |
|
With
sodium hydroxide;
In
water;
for 2h;
|
73.2% |
|
With
sodium hydroxide;
In
methanol;
at 20 ℃;
Product distribution / selectivity;
|
59.84% |
|
With
potassium hydroxide;
In
isopropyl alcohol;
at 50 - 60 ℃;
|
50% |
|
With
sodium hydroxide;
In
isopropyl alcohol;
at 39.85 ℃;
for 1h;
|
|
|
With
Amberlyst A-26 (OH);
In
methanol; water;
|
|
|
With
Amberlyst A26 (OH);
In
methanol; water;
Product distribution / selectivity;
|
|
|
With
Amberlyst A26 (OH);
In
water;
pH=~ 7 - 8;
Product distribution / selectivity;
|
|
|
With
sodium methylate;
In
methanol;
for 0.166667h;
Product distribution / selectivity;
|
|
|
With
sodium hydroxide;
In
methanol; water;
for 0.333333h;
Product distribution / selectivity;
|
|
|
With
potassium hydroxide;
In
methanol; ethanol;
at 10 - 40 ℃;
Inert atmosphere;
|
|
|
With
potassium hydroxide;
for 2h;
Product distribution / selectivity;
|
|
|
With
sodium hydroxide;
In
ethanol;
at 20 ℃;
for 1h;
|
|
|
With
sodium hydroxide;
In
water;
at 20 ℃;
for 0.5h;
|
3.18 g |
|
With
sodium hydroxide;
|
|
|
With
barium(II) hydroxide;
at 20 ℃;
for 4h;
Reagent/catalyst;
Temperature;
|
|
|
With
sodium hydroxide;
at 20 ℃;
for 0.5h;
|
1.8 g |
|
With
sodium hydroxide;
In
methanol;
at 20 ℃;
for 2h;
|
|
|
With
sodium hydroxide;
In
water; acetone;
at 20 ℃;
for 2.66667h;
Product distribution / selectivity;
|
|
|
With
sodium hydroxide;
In
ethanol;
at 20 ℃;
for 2h;
Product distribution / selectivity;
|
657-24-9 Upstream products
-
506-59-2
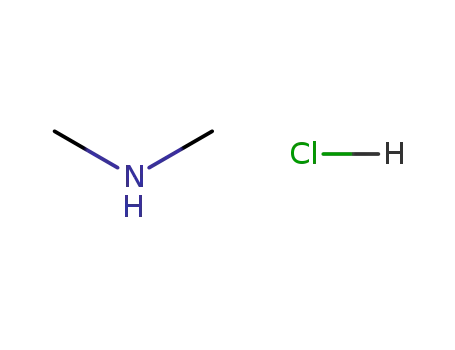
N,N-dimethylammonium chloride
-
127099-85-8
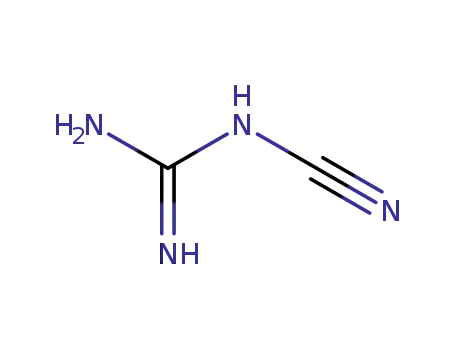
N-Cyanoguanidine
-
124-40-3
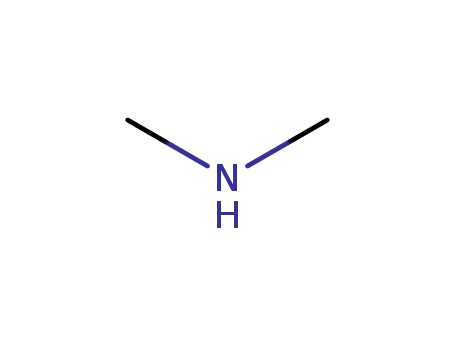
dimethyl amine
-
1115-70-4

metformin hydrochloride
657-24-9 Downstream products
-
4039-98-9
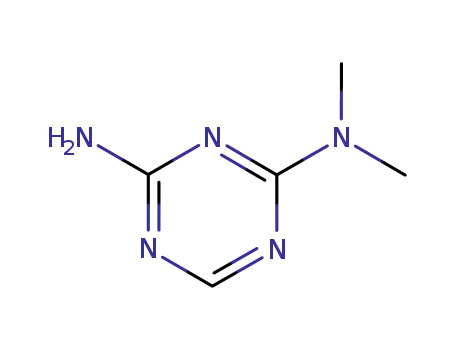
N2,N2-dimethyl-1,3,5-triazine-2,4-diamine
-
1674-64-2
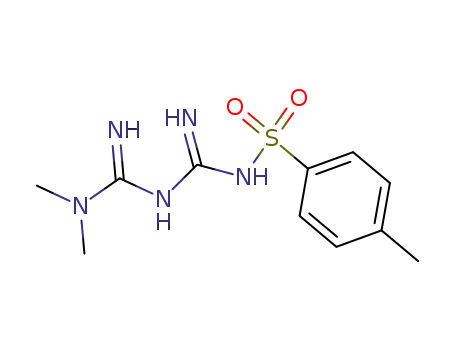
N-(4-methylphenylsulfonyl)metformin
-
201229-55-2
N-(4-Amino-6-dimethylamino-[1,3,5]triazin-2-yl)-4-chloro-2-mercapto-5-methyl-benzenesulfonamide
-
104509-76-4
N,N-dimethyl-N'-sulfanilyl-guanidine
Relevant Products
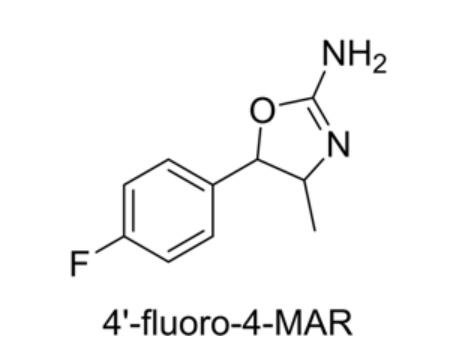
-
para-fluoro Methylaminorex
CAS:1364933-64-1
-
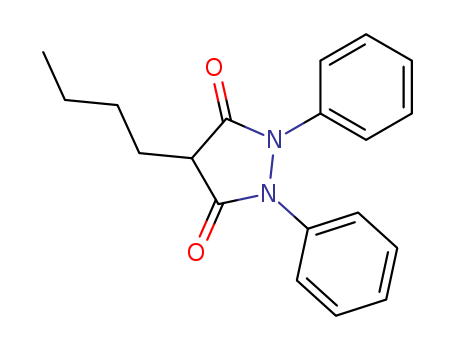
Phenylbutazone
CAS:50-33-9
-
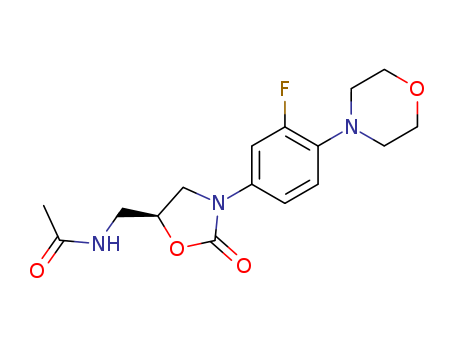
Linezolid
CAS:165800-03-3