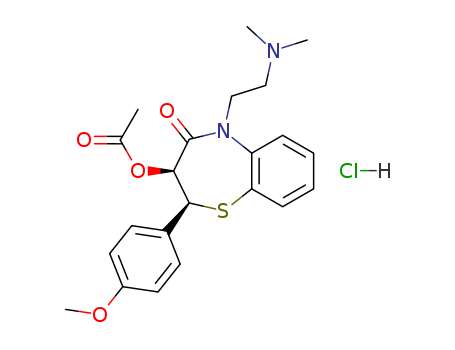
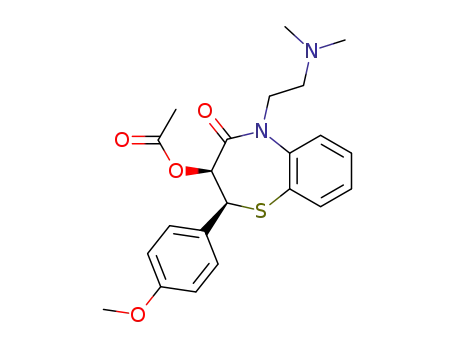
33286-22-5
- Product Name:Diltiazem hydrochloride hcl
- Molecular Formula:C22H26N2O4S.HCl
- Purity:99%
- Molecular Weight:450.986
Product Details;
CasNo: 33286-22-5
Molecular Formula: C22H26N2O4S.HCl
Appearance: Fine needles
Manufacturer supply Diltiazem hydrochloride hcl 33286-22-5 with sufficient stock and high standard
- Molecular Formula:C22H26N2O4S.HCl
- Molecular Weight:450.986
- Appearance/Colour:Fine needles
- Vapor Pressure:4.27E-14mmHg at 25°C
- Melting Point:212-214 ºC
- Refractive Index:118 ° (C=1, H2O)
- Boiling Point:594.4 ºC at 760 mmHg
- Flash Point:313.3 ºC
- PSA:84.38000
- Density:1.26g/cm3
- LogP:4.23550
Dilthiazem hydrochloride(Cas 33286-22-5) Usage
|
Manufacturing Process |
β-Diethylaminoethyl chloride is condensed with 2-(4-methoxyphenyl)-3- hydroxy-2,3-dihydro-1,5-benzothiazepin-4(5H)-one in a first step. Then a mixture of 1.5 grams of 2-(4-methoxyphenyl)-3-hydroxy-5-(βdimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one and 20 ml of acetic anhydride was heated on a water bath for 5 hours. The reaction mixture was evaporated under reduced pressure to remove acetic anhydride and the concentrated product was poured into ice water. The resulting mixture was made alkaline with sodium bicarbonate and extracted with chloroform. The chloroform layer was dried and evaporated to remove the solvent. The residue was dissolved in acetone, and an ethanol solution containing hydrogen chloride was added thereto producing 1.53 grams of 2-(4-methoxyphenyl)3- acetoxy-5-(β-dimethylaminoethyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one hydrochloride having a melting point from 187° to 188°C.The starting material is made by reacting 4-methoxybenzaldehyde with ethyl chloroacetate; that product with sodium ethoxide; and that product with 2- aminothiophenol. |
|
Therapeutic Function |
Coronary vasodilator |
|
Biological Activity |
Antihypertensive and cardioprotective agent; an inhibitor of L-type Ca 2+ channels. |
|
Biochem/physiol Actions |
Product does not compete with ATP. |
|
Metabolism |
Diltiazem is almost completely absorbed from the gastrointestinal tract after oral doses, but undergoes extensive first-pass hepatic metabolism resulting in a bioavailability of about 40%. It is extensively metabolised in the liver, mainly by the cytochrome P450 isoenzyme CYP3A4; one of the metabolites, desacetyldiltiazem, has been reported to have 25-50% of the activity of the parent compoundAbout 2-4% of a dose is excreted in urine as unchanged diltiazem with the remainder excreted as metabolites in bile and urine. |
|
Mode of action |
Diltiazem Hydrochloride is a benzothiazepine calcium channel blocking agent. Diltiazem hydrochloride inhibits the transmembrane influx of extracellular calcium ions into select myocardial and vascular smooth muscle cells, causing dilatation of coronary and systemic arteries and decreasing myocardial contractility. Because of its vasodilatory activity, this agent has been shown to improve the microcirculation in some tumors, thereby potentially improving the delivery of antineoplastic agents to tumor cells. |
|
Definition |
ChEBI: Diltiazem hydrochloride is a hydrochloride salt resulting from the reaction of equimolar amounts of diltiazem and hydrogen chloride. A calcium-channel blocker and vasodilator, it is used in the management of angina pectoris and hypertension. It has a role as an antihypertensive agent, a vasodilator agent and a calcium channel blocker. It contains a diltiazem(1+). It is an enantiomer of an ent-diltiazem hydrochloride. |
|
Brand name |
Cardizem (Biovail); Cartia (Andrx); Dilacor (Watson); Diltzac (Apotex); Taztia (Andrx); Tiazac (Biovail). |
|
General Description |
Diltiazem hydrochloride is a calcium ion cellular influx inhibitor. It was developed and introduced inJapan as a cardiovascular agent to treat angina pectoris. Itwas observed to dilate peripheral arteries and arterioles. Thedrug increases myocardial oxygen supply by relieving coronaryartery spasm and reduces myocardial oxygen demandby decreasing heart rate and reducing overload. Diltiazemhydrochloride is used in patients with variant angina. Thedrug has electrophysiological properties similar to those ofverapamil and is used in clinically similar treatment conditionsas an antiarrhythmic agent, but it is less potent.The drug is absorbed rapidly and almost completely fromthe digestive tract. It reaches peak plasma levels within 1hour after administration in gelatin capsules. Oral formulationson the market are sustained-release preparations providingpeak plasma levels 3 to 4 hours after administration. |
InChI:InChI=1/C22H26N2O4S.ClH/c1-15(25)28-20-21(16-9-11-17(27-4)12-10-16)29-19-8-6-5-7-18(19)24(22(20)26)14-13-23(2)3;/h5-12,20-21H,13-14H2,1-4H3;1H/t20-,21+;/m1./s1
33286-22-5 Relevant articles
PROCESS FOR PREPARING DILTIAZEM USING A HETEROGENEOUS TRIFUNCTIONAL CATALYST
-
Page 7-8, (2008/06/13)
The present invention comprises a simpli...
Process for preparing diltiazem using a heterogeneous trifunctional catalyst
-
Page 4, (2008/06/13)
The present invention comprises a simpli...
Improved procedure for Julia-Colonna asymmetric epoxidation of α,β-unsaturated ketones: Total synthesis of diltiazem and Taxol side-chain
Adger, Brian M.,Barkley, James V.,Bergeron, Sophie,Cappi, Michael W.,Flowerdew, Benjamin E.,Jackson, Mark P.,McCague, Ray,Nugent, Thomas C.,Roberts, Stanley M.
, p. 3501 - 3507 (2007/10/03)
Poly-L-leucine catalyses the asymmetric ...
Process for the preparation of diltiazem
-
, (2008/06/13)
(+)-Cis-3-(acetoxy)-5-[2-(dimethylamino)...
33286-22-5 Process route

-
-
42399-41-7
diltiazem

-
-
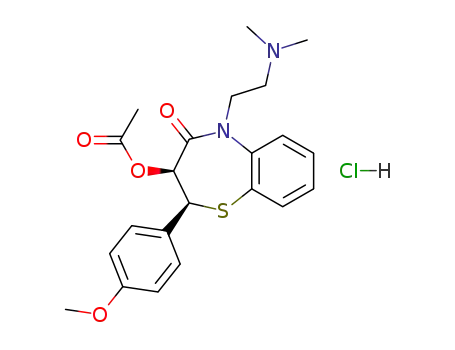
33286-22-5
diltiazem hydrochloride
| Conditions | Yield |
|---|---|
|
|
|
|
|
|
|
|
-
-
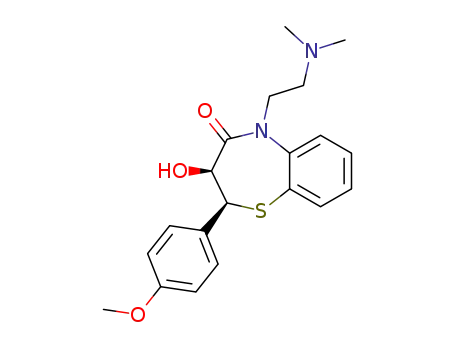
42399-40-6
O-desacetyldiltiazem

-
-
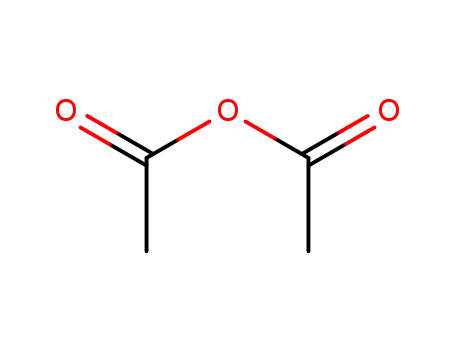
108-24-7
acetic anhydride

-

-
33286-22-5
diltiazem hydrochloride
| Conditions | Yield |
|---|---|
|
O-desacetyldiltiazem; acetic anhydride;
With
dmap; triethylamine;
In
dichloromethane;
for 3h;
Heating / reflux;
With
hydrogenchloride;
In
methanol;
pH=2;
|
92% |
|
With
hydrogenchloride;
In
ethanol; butanone;
at 90 ℃;
for 1h;
|
80% |
|
With
dmap;
In
dichloromethane;
for 3h;
Yield given;
Heating;
|
33286-22-5 Upstream products
-
108-22-5
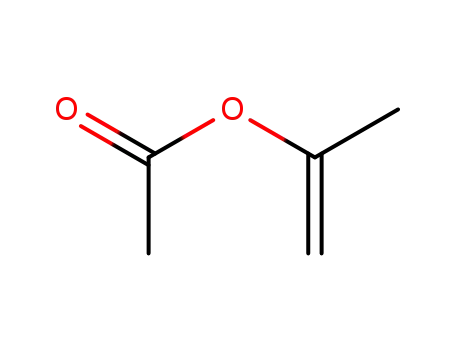
Isopropenyl acetate
-
75472-91-2
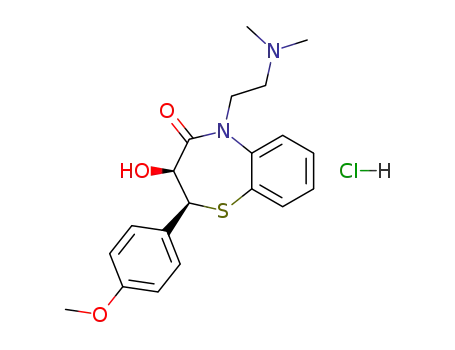
(2S,3S)-5-(2-dimethylaminoethyl)-3-hydroxy-2-(4-methoxyphenyl)-2,3-dihydro-1,5-benzothiazepin-4(5H)-one hydrochloride
-
42399-40-6

O-desacetyldiltiazem
-
108-24-7

acetic anhydride
33286-22-5 Downstream products
-
42399-41-7

diltiazem
-
23515-44-8
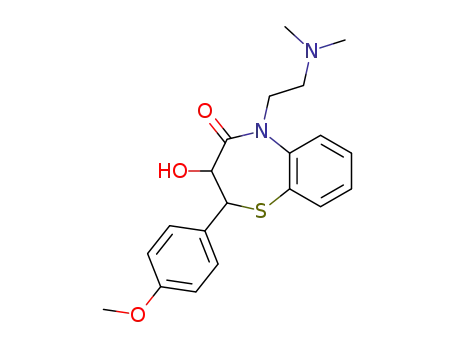
cis(+)-3-hydroxy-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-1,5-benzothiazepin-4(5H)-one
-
42399-40-6

O-desacetyldiltiazem
Relevant Products
-
Quinine
CAS:130-95-0
-
Polyethylene glycol monomethallyl ether
CAS:31497-33-3